Anti-inflammatory polypeptide nano drug and preparation method thereof
A nano-drug and responsive technology, applied in anti-inflammatory agents, drug combinations, pharmaceutical formulations, etc., can solve the problems of short half-life of polypeptide drugs, limited clinical application, and inability to take oral administration, etc., and achieves easy large-scale preparation and low price , good in vivo and in vitro safety effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
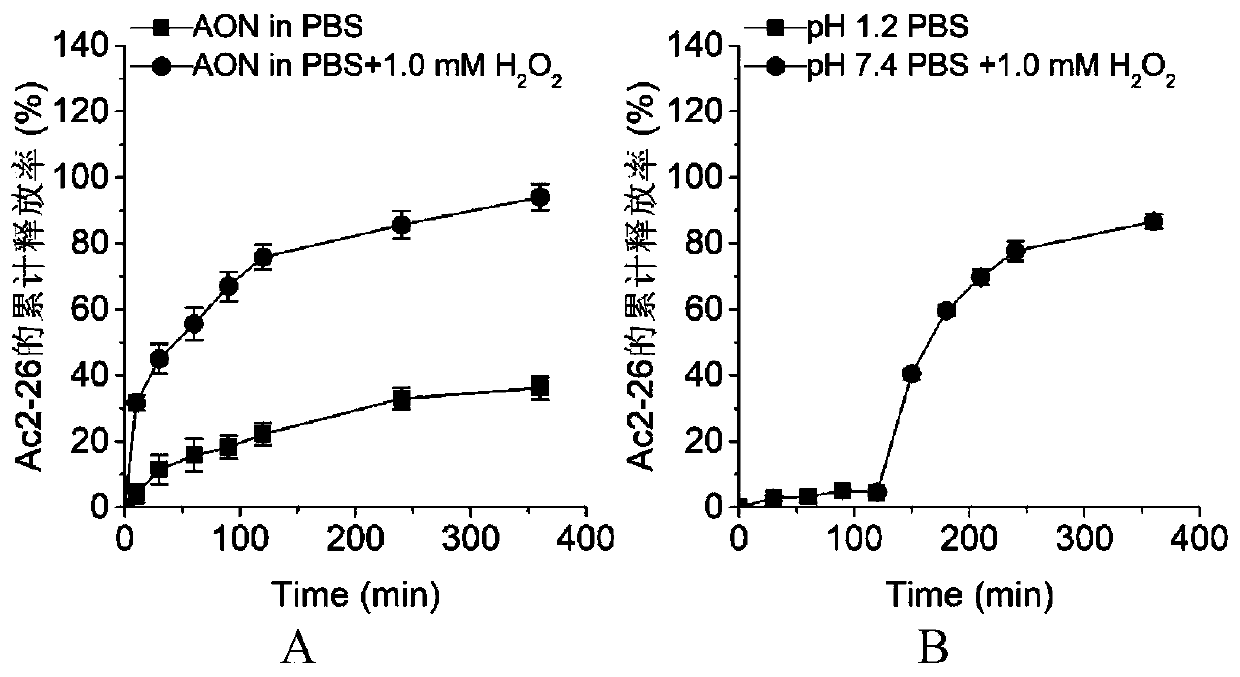
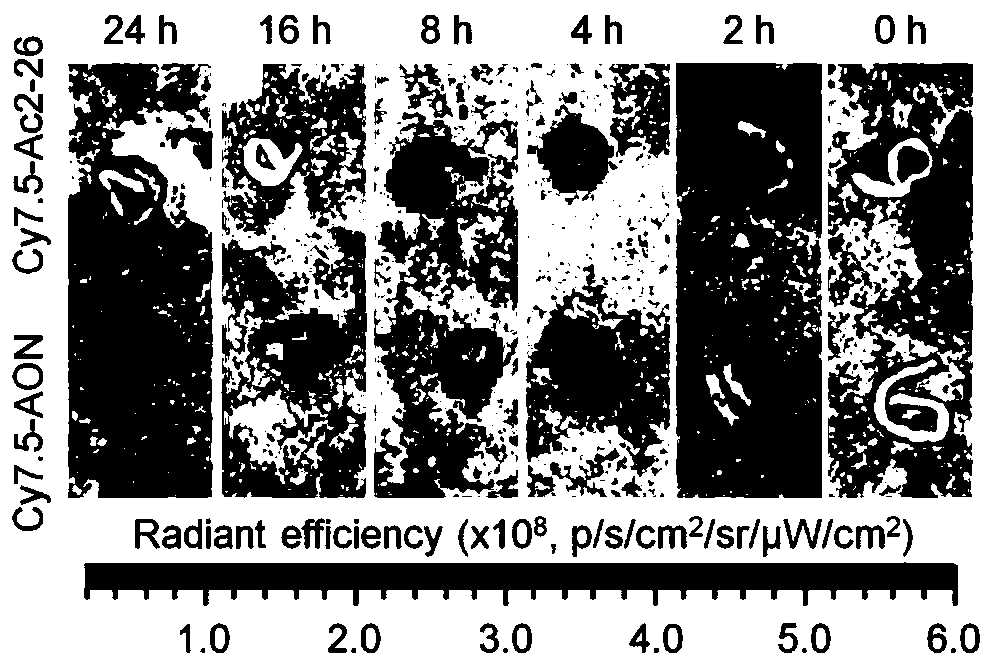
[0040] First, 2mg lecithin and 15mg polyethylene glycol-distearoylphosphatidylethanolamine (the molecular weight of polyethylene glycol is 2000Da) are dissolved in 2mL deionized water under the condition of constant temperature magnetic stirring at 65℃. Dissolve completely, let it cool to room temperature. 50 mg of acetalized α-cyclodextrin was dissolved in 4 mL of methanol. 1 mg Ac2-26 was dissolved in 100 μL of deionized water and added to the above organic phase. Then, while stirring, the organic phase was slowly added dropwise to the water phase (1 mL / min). After the addition is complete, continue stirring at 25°C for 3 hours. After centrifugal separation, washing with deionized water, and freeze-drying, the pH-responsive nanomedicine of the present invention can be obtained. The particle size of nano-medicine is between 100-200nm.
Embodiment 2
[0042] First, 1mg lecithin and 5mg polyethylene glycol-distearoylphosphatidylethanolamine (the molecular weight of polyethylene glycol is 2000Da) are dissolved in 2mL deionized water under the condition of constant temperature magnetic stirring at 65℃. Dissolve and leave to cool to room temperature. 30 mg of acetalized β-cyclodextrin was dissolved in 3 mL of methanol / acetonitrile. 1 mg Ac2-26 was dissolved in 100 μL of deionized water and added to the above organic phase. Then, while stirring, the organic phase was slowly added dropwise to the water phase (1 mL / min). After the addition is complete, continue stirring at 35°C for 4 hours. After centrifugal separation, washing with deionized water, and freeze-drying, the pH-responsive nanomedicine of the present invention can be obtained. The particle size of nanomedicine is between 20-150nm.
Embodiment 3
[0044] First, 0.5 mg of lecithin and 5 mg of polyethylene glycol-distearoyl phosphatidyl ethanolamine (the molecular weight of polyethylene glycol is 2000 Da) are dissolved in 2 mL of deionized water under the condition of constant temperature magnetic stirring at 65°C. Dissolve and leave to cool to room temperature. 40 mg of acetalized γ-cyclodextrin was dissolved in 5 mL methanol / tetrahydrofuran. 1 mg Ac2-26 was dissolved in 100 μL of deionized water and added to the above organic phase. Then, while stirring, the organic phase was slowly added dropwise to the water phase (1 mL / min). After the addition is complete, continue stirring at 45°C for 1 hour. After centrifugal separation, washing with deionized water, and freeze-drying, the pH-responsive nanomedicine of the present invention can be obtained. The particle size of nanomedicine is between 50-250nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com