Targeting Pseudotyped Retroviral Vectors
a technology of pseudotyped retroviral vectors and targeted genes, applied in the field of lentiviral vectors, can solve the problems of limited in vivo usefulness of lentiviral vectors, difficult to apply specific targeting to retroviral vectors, and few studies of retroviral vector targeting in living animals are not efficient, so as to reduce the natural tropism of sindbis virus envelope, increase the specificity of targeted gene transduction, and reduce the endogenous tropism of e2
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
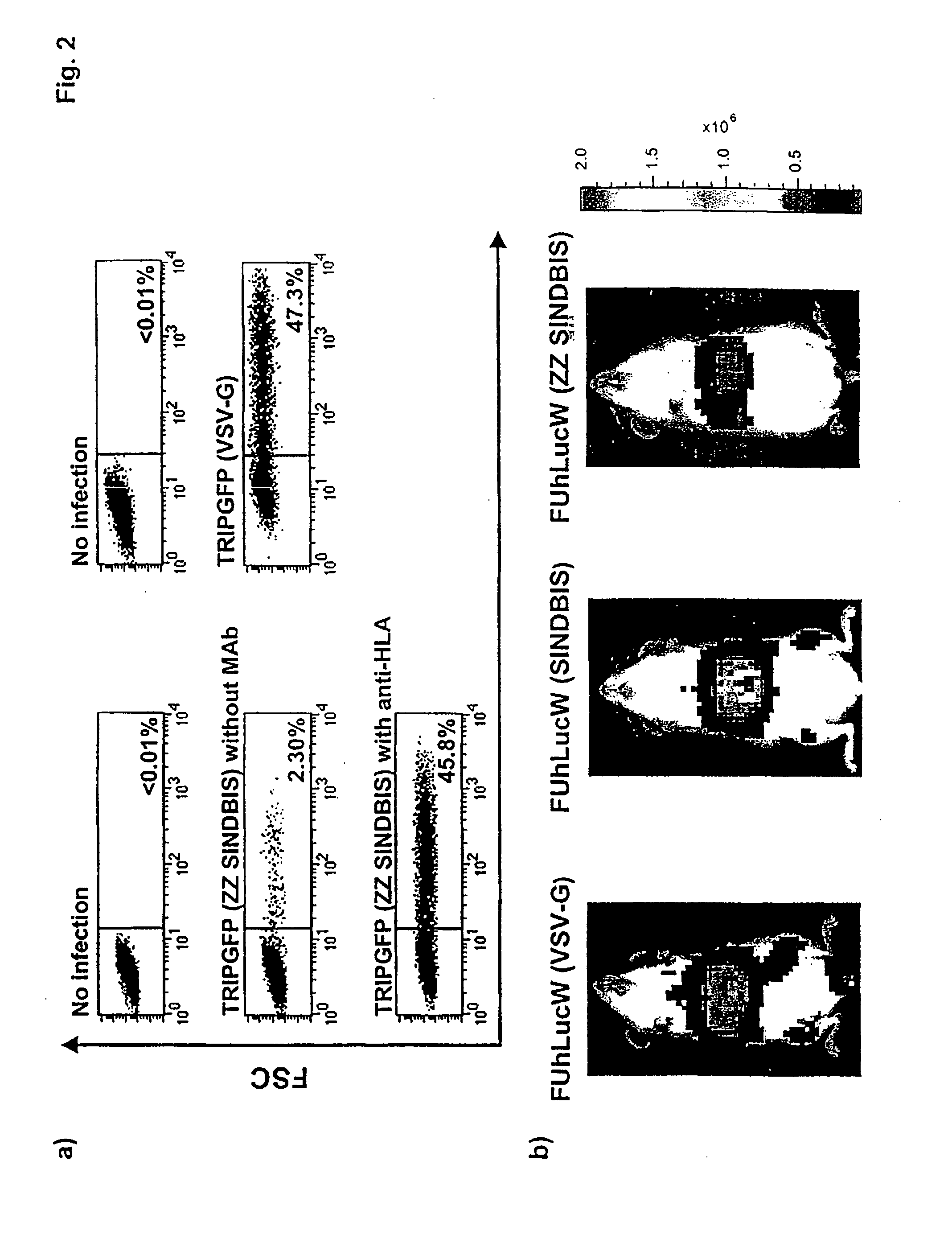
Targeted Lentiviral Vectors with Mutated Sindbis Envelopes
Methods
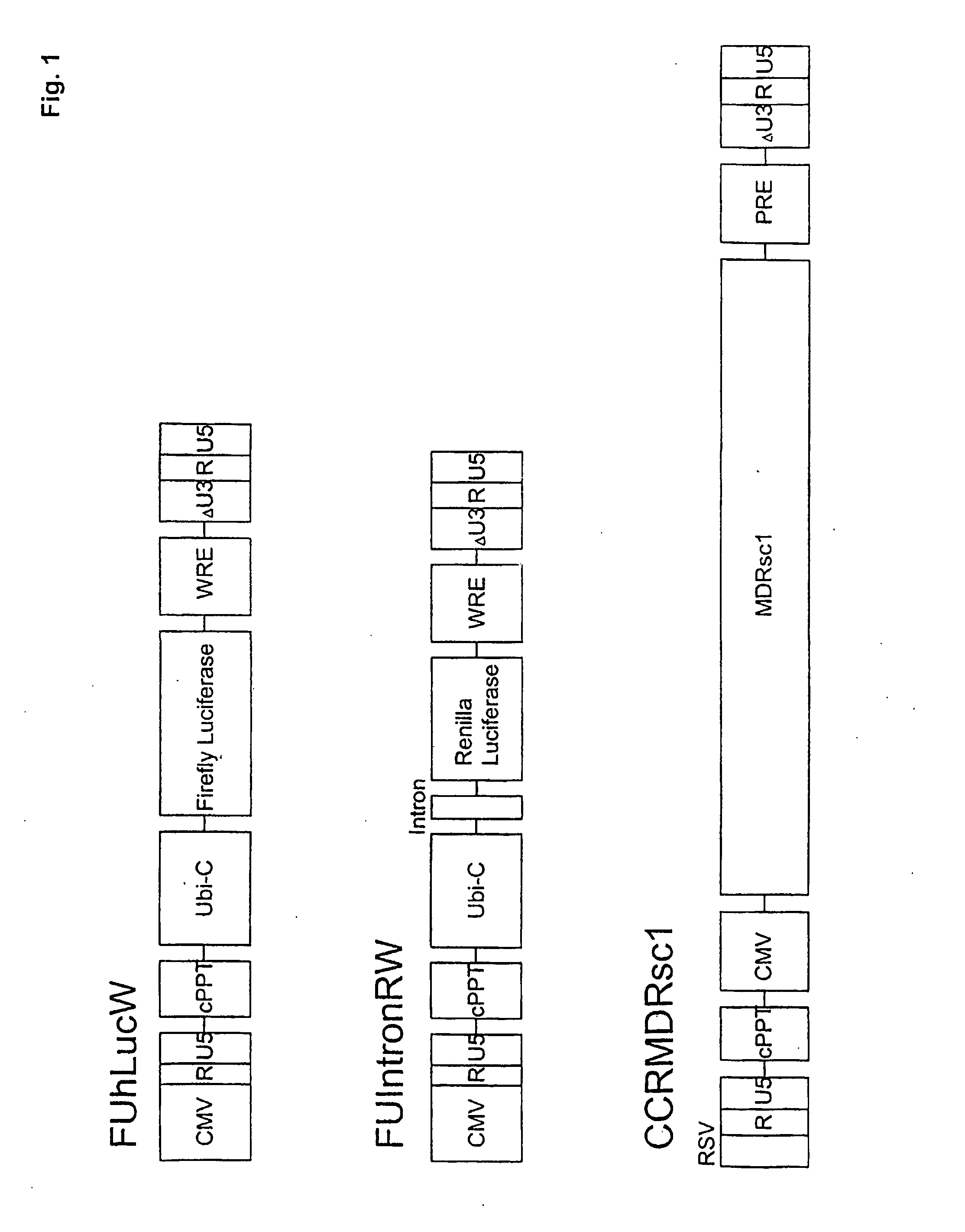
[0099]Plasmid construction. All mutants of pIntron ZZ SINDBIS were generated using a site directed mutagenesis kit (Stratagene, La Jolla, Calif.). Initially, the envelope region of pIntronZZ SINDBIS was cloned into pBS-SKII. Mutagenesis was performed using various oligonucleotide corresponding to the mutations (Table 1) following the manufacturer's protocol. The mutations were confirmed by sequence analysis. The sequenced regions were then cloned back into pIntron ZZSINBIS. CCR MDRsc1 was constructed from pRRL-cPPTCMV-X-PRE (kindly provided by Dr. William Osborne) (Barry et al., Hum. Gene Ther. 12, 1103-1108 (2001)) and ha-MDRsc (kindly provided by Dr. Brian Sorrentino) (Bunting et al., Blood 92, 2269-2279 (1998)). FUhLucW was constructed from FUGW (kindly provided by Dr. David Baltimore) and pGL3-Basic (Promega, Madison, Wis.). FUIntronRW was constructed from FUGW and phRL-CMV (Promega).
TABLE 1Sindbis Envelope Mutatio...
example 2
Targeting Prostate Cancer Cells through the Prostate Stem Cell Antigen (PSCA)
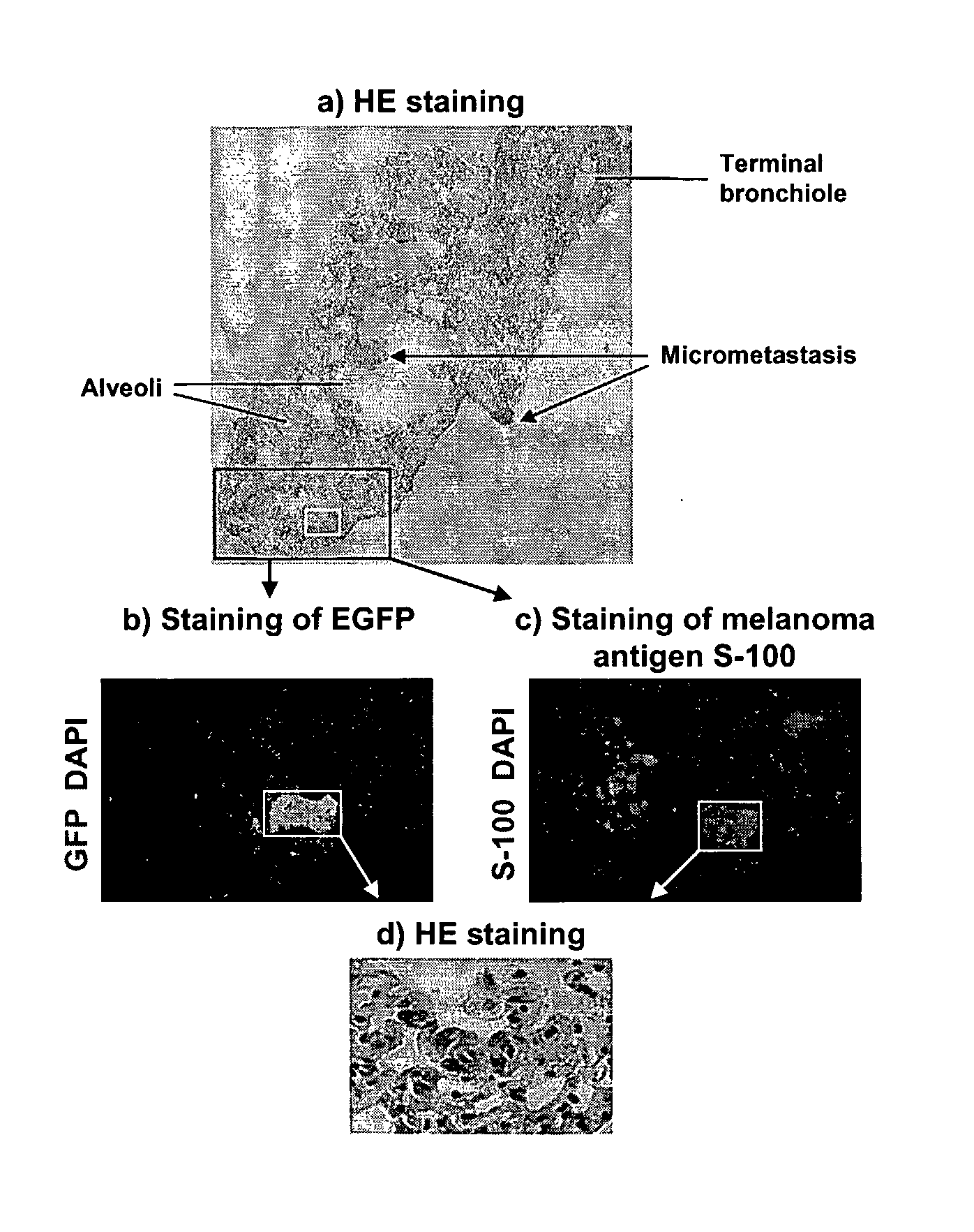
[0133]Prostate cancer is the most common cancer diagnosis and the second leading cause of cancer-related death in American men. Prostate cancer mortality frequently results from metastasis to bone and hormone-independent tumor growth. Physiologically relevant prostate cancer models exist, such as the LAPC-9 xenograft model (see, Craft, et al., Cancer Res (2000) 60:2541-2546). LAPC-9 cells were derived from a human bone metastasis, and are able to form a prostate cancer xenograft that can be propagated in SCID mice, and expresses prostate specific antigen and wild-type androgen receptor (Id.). Hormone independent outgrowths can be selected after castration of the mice, and micrometastasis can eventually be detected in half of the mice, recapitulating the clinical progression of human prostate cancer (Klein, et al., Nature Med (1997) 3:402-408).
[0134]We generated vectors that specifically deliver genes to pro...
example 3
Combining Cell Surface Targeting with Selective Cell to Increase Efficiency of Specific Targeting
[0135]The ubiquitin-C promoter in the FUGW lentiviral vector (Morizono, et al., Nature Medicine (2005) 11:346-352; and Lois, et al., Science (2002) 295:868-872), with a chimeric prostate specific antigen (PSA) promoter, designated (PSE-BC), to produce the construct FPGW. The key regulatory elements of the PSA enhancer include a proximal promoter (−541 to +12) comprising two binding sites for the androgen receptor (AREI and II) and a distal enhancer, which contains a 390-bp androgen responsive core region (Schuur, et al., J Biol Chem (1996) 8:1416-1426; and Cleutjens, et al., J Biol Chem (1996) 271:6379-6388). The core region contains a cluster of closely spaced androgen response elements (AREs) and sites for other transcription factors. The androgen receptor (AR) binds cooperatively to the enhancer and mediates synergistic transcription, and other factors within and outside of the enhanc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tm | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com