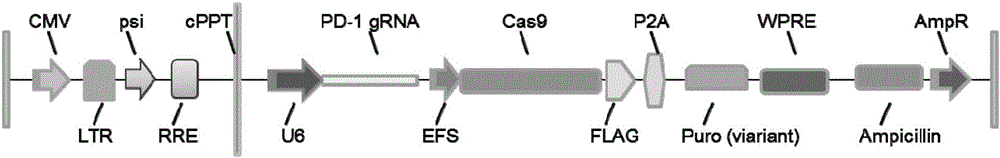
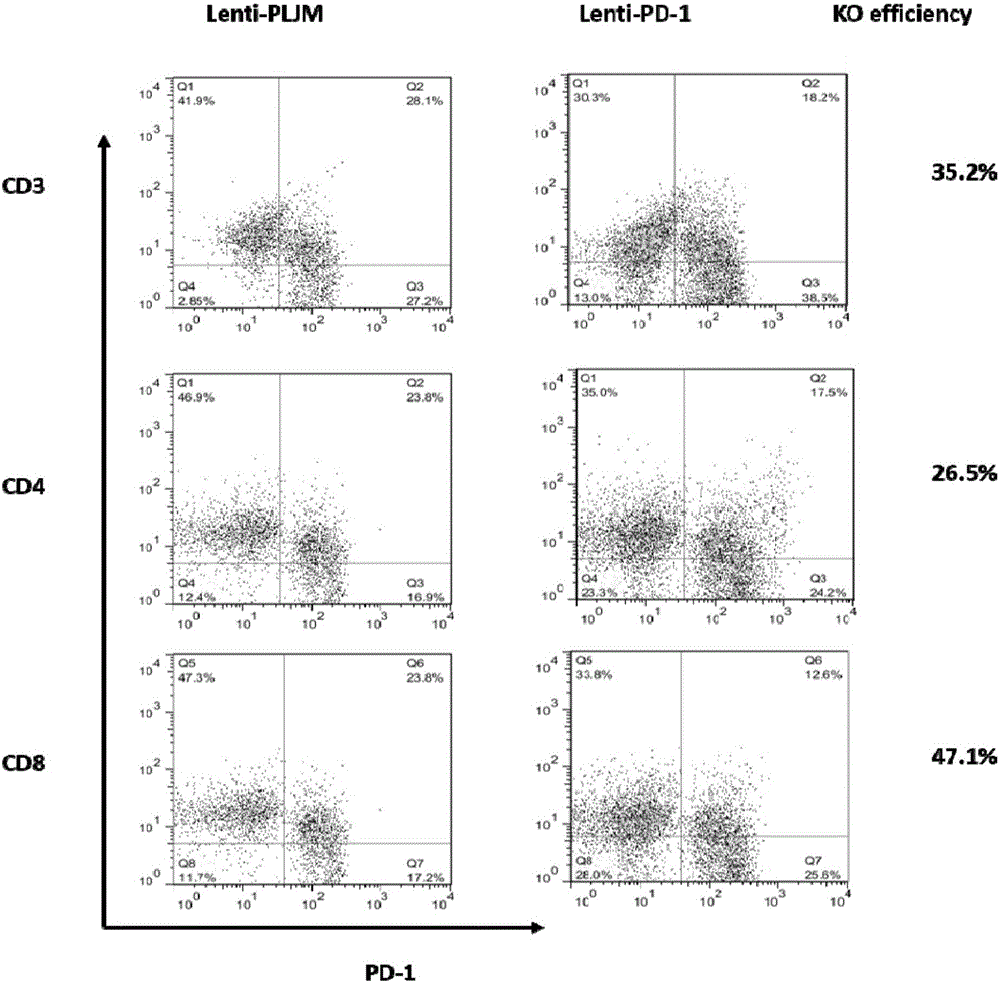
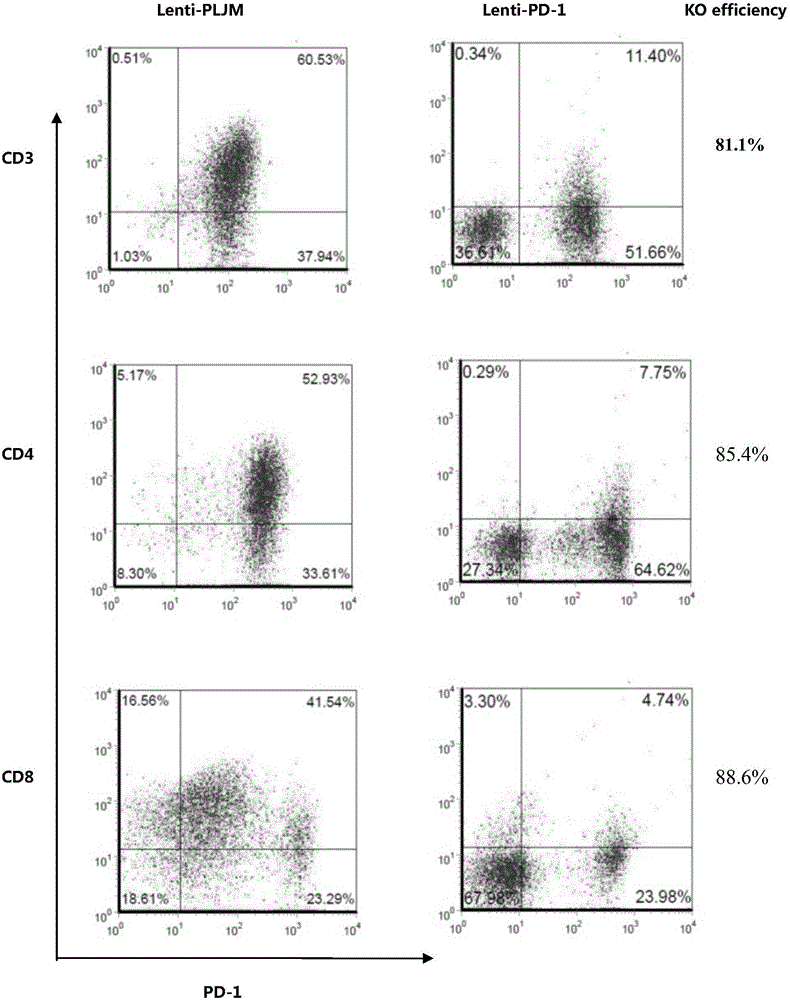
PD-1 gene recombinant virus plasmid, construction thereof, recombinant retrovirus Lenti-PD-1-Puro and packaging and application of recombinant retrovirus Lenti-PD-1-Puro
A cas9-pd-1-puro and retrovirus technology, applied in the direction of retroRNA virus, application, virus, etc., can solve the problems of complex antibody preparation and purification process, expensive antibody drugs, high manufacturing cost, etc., and achieve specificity Strong, low cost, easy to culture and expand the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 (preparation of PD-1 gene recombinant virus)
[0036] 1) Dephosphorylate the Lenti-CRISPR / Cas9 plasmid (Addgene52961) with EcoR1, Age1 endonuclease and phosphatase at 37°C for 30 minutes to obtain the Lenti-CRISPR / Cas9-Puro plasmid;
[0037] 2) Using a total of 20 base sequences at positions 2859-2878 in the PD-1 gene sequence as the PD-1-specific guide RNA, that is, PD-1gRNA, under the action of T4 ligase (NEBM2200S), the PD-1gRNA primer sequence The complementary strand was incubated at 37°C for 30 minutes, incubated at 95°C for 5 minutes, and then annealed at a rate of 5°C per minute to 25°C to synthesize PD-1 double-stranded DNA;
[0038] 3) Use fast nucleic acid ligase T4 ligase (NEBM2200S) to connect the PD-1 double-stranded DNA to the Lenti-CRISPR / Cas9-Puro plasmid obtained in step 1), and incubate at room temperature for 10 minutes to obtain the recombinant viral plasmid Lenti-CRISPR / Cas9-Puro Cas9-PD-1-Puro;
Embodiment 2
[0039] Embodiment 2 (packaging of recombinant retrovirus Lenti-PD-1-Puro)
[0040] 1) Transfer the recombinant virus plasmid Lenti-CRISPR / Cas9-PD-1-Puro into Stbl3 bacteria, screen with ampicillin, amplify, purify, and sequence, as follows:
[0041] Screening: place the bacterial species transferred into the plasmid on an agar plate containing ampicillin (100 μg / ml), incubate at 37° C. for 12 hours and grow 10 to 20 colonies, and select 3 to 5 colonies to amplify;
[0042] Amplification: Put the above-mentioned selected colonies into 300 ml of LB bacterial culture solution (containing ampicillin, 100 μg / ml), and incubate on a shaker at 37°C for 16 hours, and the bacteria will be amplified in large quantities;
[0043] Purification: Purify with the plasmid extraction kit (Cat. No. 12162) from Qiagen, USA, to obtain 1 to 2 mg of viral plasmid;
[0044] Sequencing: The proposed plasmid was sent to a sequencing company (Laragen, USA) for sequencing, and the viral plasmid with 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com