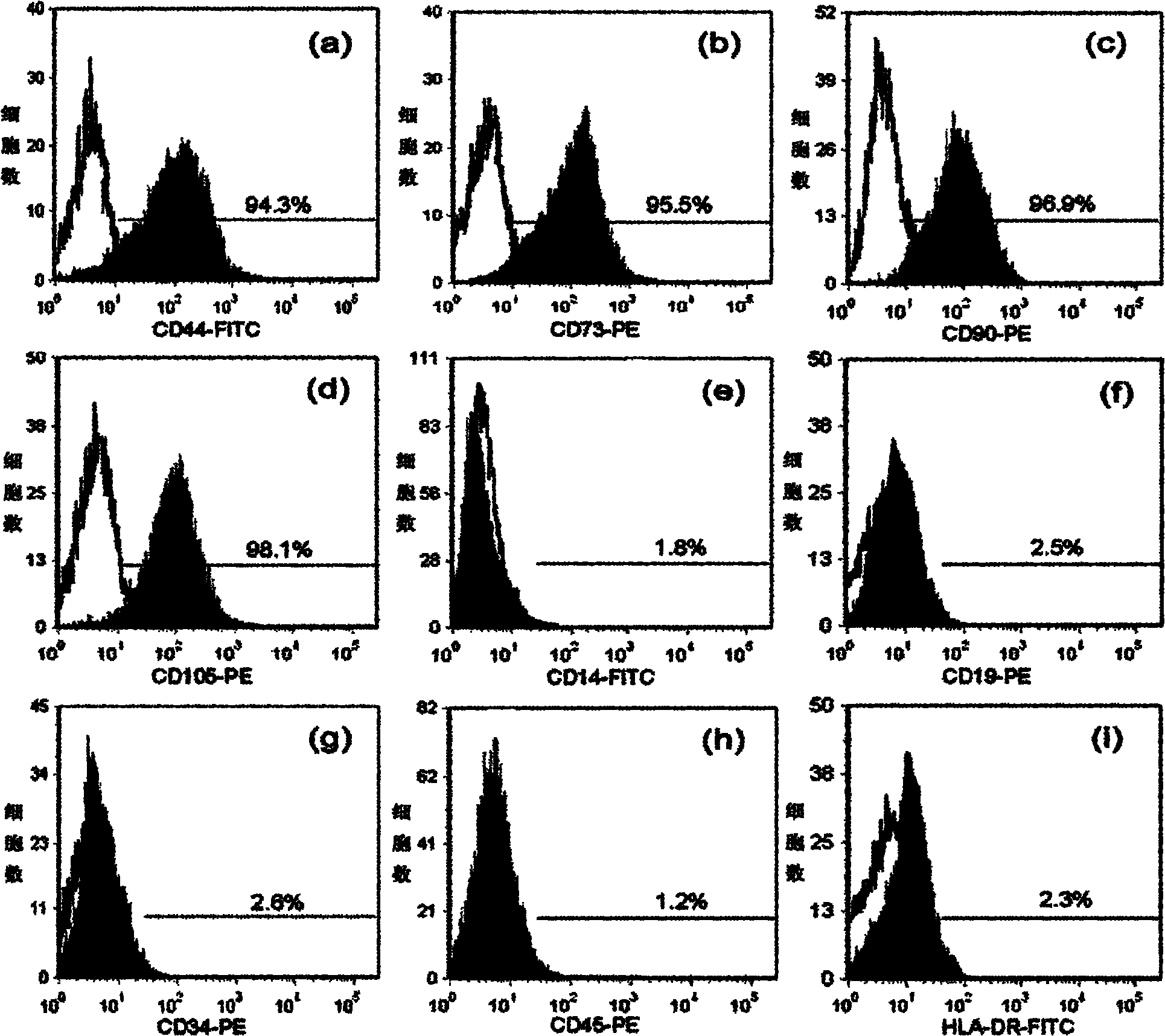
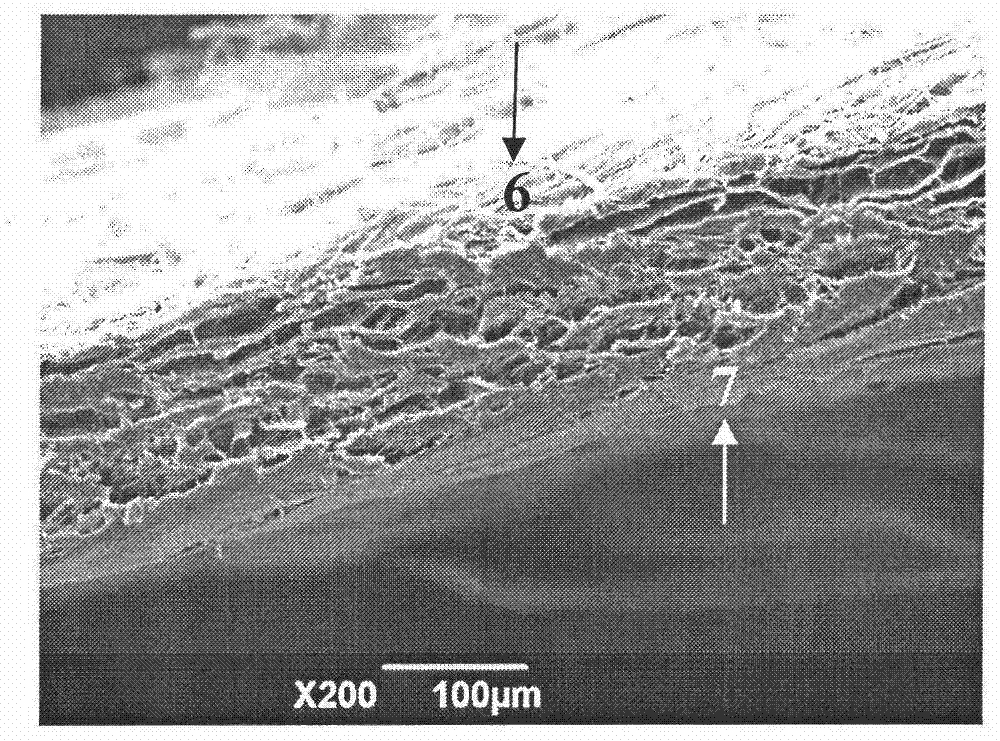
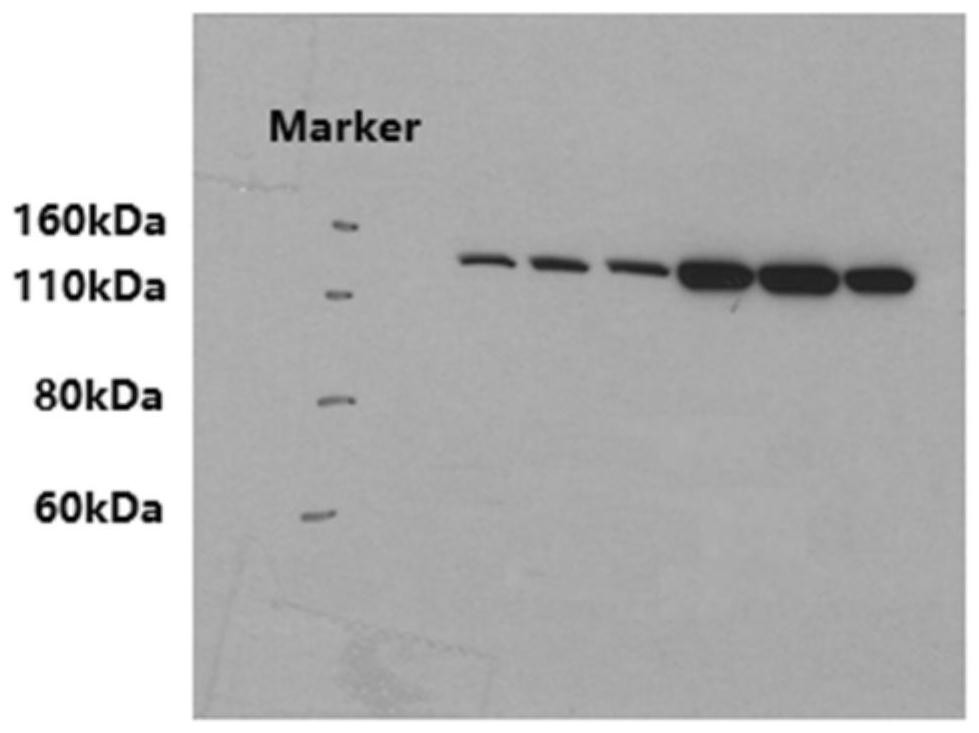
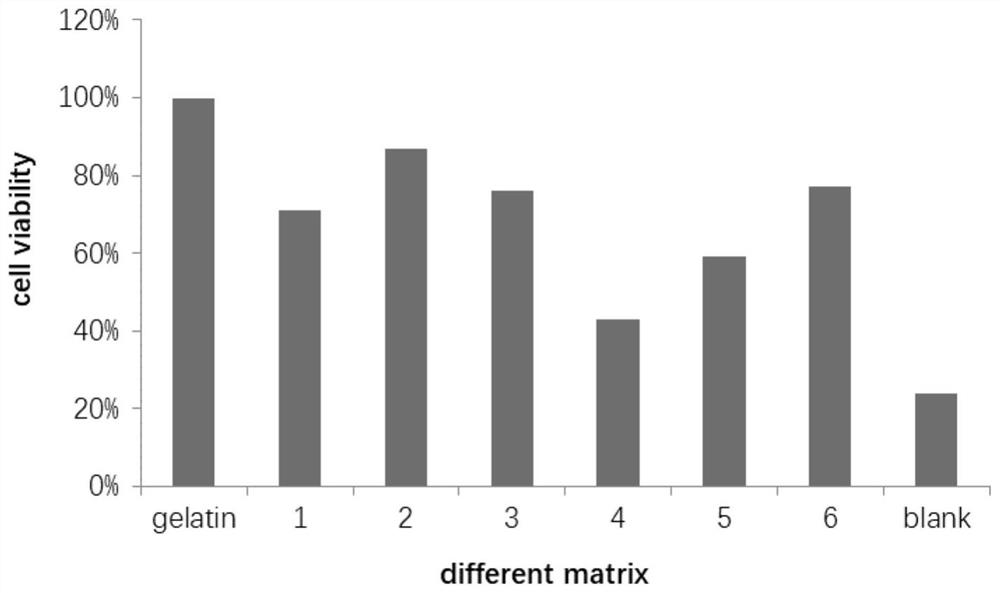
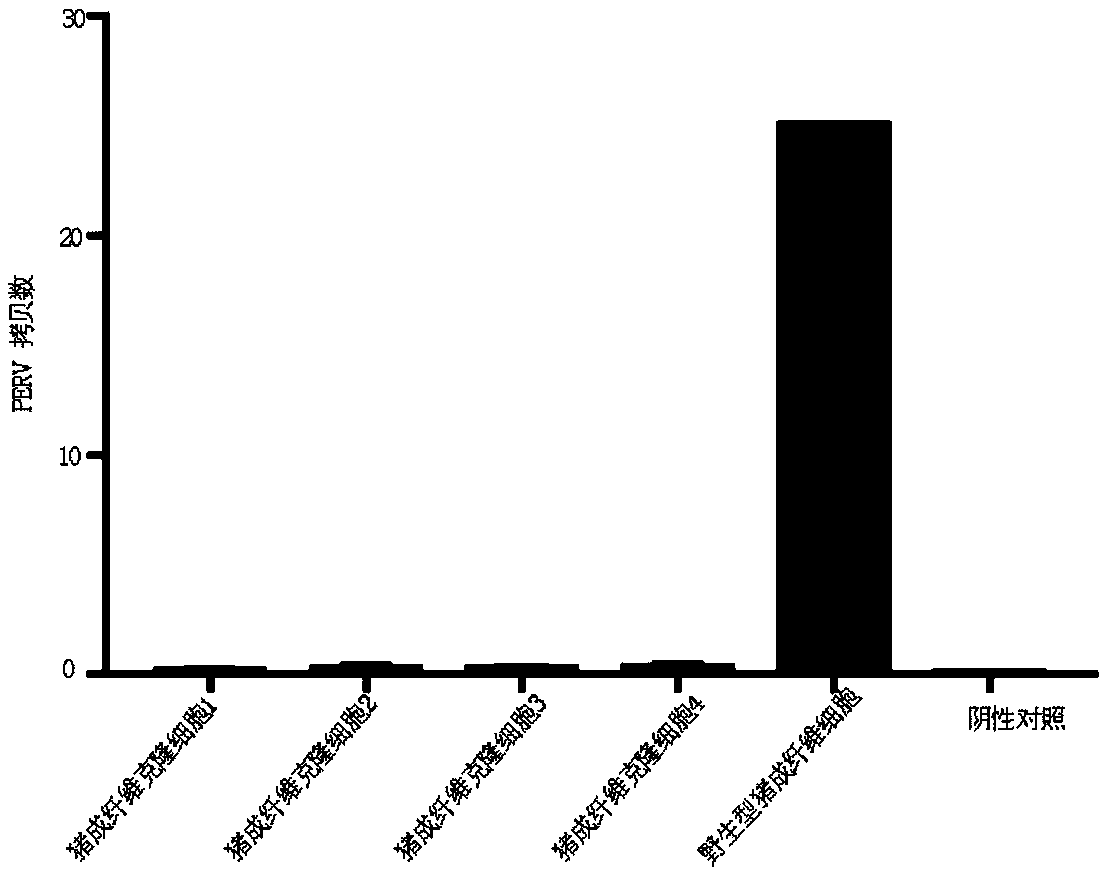
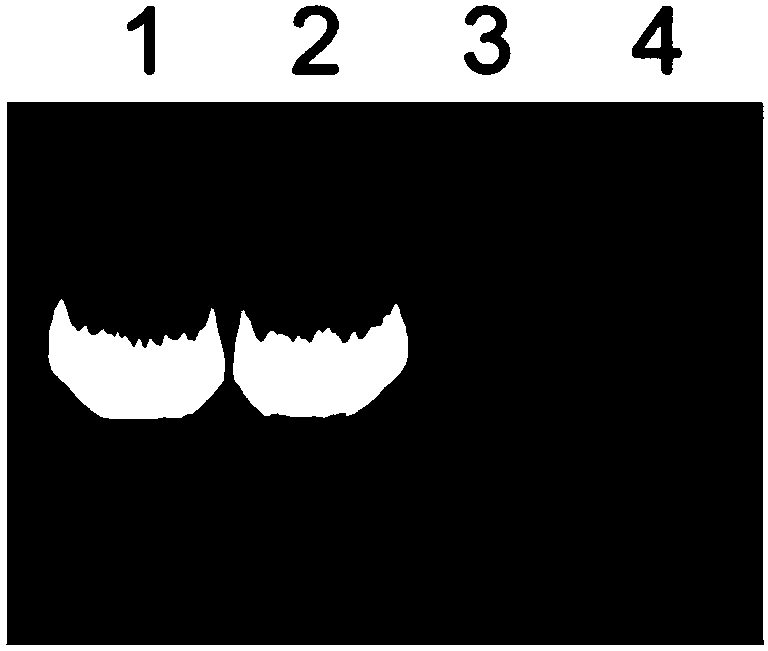
Patents
Literature
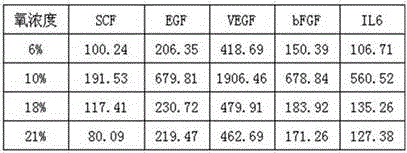
168results about How to "No immune rejection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical anti-sticking membrane and preparation method thereof
The invention provides a medical anti-sticking membrane and a preparation method thereof. The anti-sticking membrane comprises a nanometer frame and hydrosol attached thereon. The hydrosol is internally packed with one or several kinds of styptic medicament or / and anti-sticking medicament. The invention also provides a preparation method of the anti-sticking membrane, comprising the following steps of: preparing electro-spinning solution, styptic medicament and / or anti-sticking medicament-containing hydrosol solution and crosslinker solution; receiving static spinning with the crosslinker solution to obtain the nanometer frame; printing the styptic medicament and / or anti-sticking medicament-containing hydrosol solution onto the nanometer frame by an ink-jet printer, and solidifying the hydrosol solution to obtain the anti-sticking membrane. The anti-sticking membrane has good capability and biological compatibility, and nontoxicity as well as nonirritant, can be completely degraded and absorbed, is compounded with controllably released styptic medicament or / and anti-sticking medicament, has controllable degrading time and speed, and conquers defects of the prior art.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
Nano bionic wound-surface cover and preparation method thereof
ActiveCN101507835AAddress barriers to developmentAvoid inconvenienceProsthesisElectrospinningEngineering
The invention provides a nanometer bionic wound-surface cover and a preparation method thereof. The nanometer bionic wound-surface cover comprises a nanometer bionic bracket and hydrosol attached to the bracket, wherein the hydrosol covers one or a plurality of cytokines. The preparation method for the nanometer bionic wound-surface cover provided by the invention comprises the steps of preparingan electrostatic-spinning solution, a cytokine-containing hydrosol solution and a crosslinker solution, preparing the nanometer bionic bracket by use of electrostatic spinning, using an ink-jet printer to print the cytokine-containing hydrosol solution onto the nanometer bionic bracket, and the like, wherein electrostatic spinning and printing can be repeated so as to form the wound-surface covers different in thickness. The preparation method adopts an in-situ autologous stem-cell engineering technique and adopts stem-cell chemotactic factors to attract autologous stem cells to directionally migrate, enter a wound surface and be differentiated according to designed requirements, thereby avoiding inconvenience caused by using viable cells, achieving rehabilitation effects the same with orbetter than that of using the viable cells and having broad application prospects.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
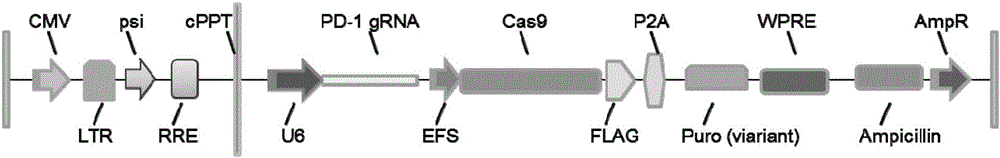
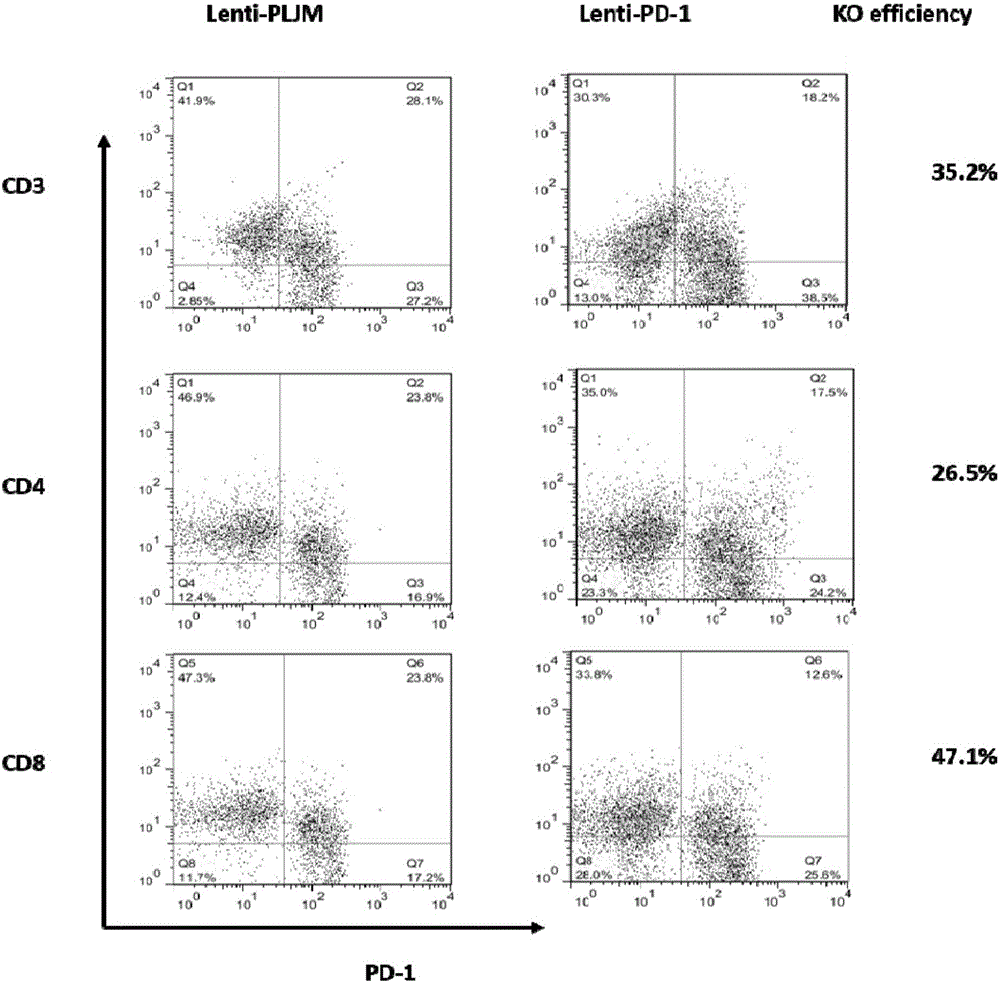
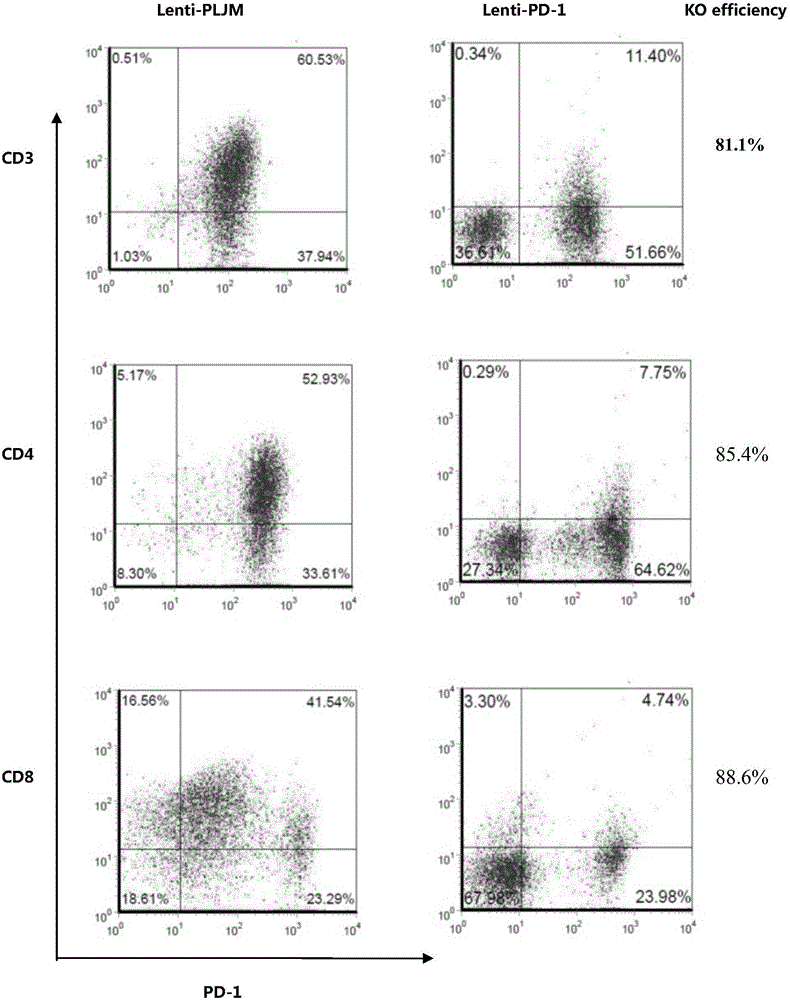
PD-1 gene recombinant virus plasmid, construction thereof, recombinant retrovirus Lenti-PD-1-Puro and packaging and application of recombinant retrovirus Lenti-PD-1-Puro
ActiveCN105671083AStrong specificityRelease the suppressed stateCell receptors/surface-antigens/surface-determinantsUnknown materialsT cellViral infection
The invention discloses a PD-1 gene recombinant virus. The collection number of the recombinant virus Lenti-PD-1-Puro is CCTCC No:V201601. According to the PD-1 gene recombinant virus, PD-1gRNA sequences are cloned into a retrovirus plasmid Lenti-CRISPR / Cas9-Puro, and a PD-1 recombinant virus plasmid Lenti-CRISPR / Cas9-PD-1-Puro is obtained; then the PD-1 recombinant virus plasmid Lenti-CRISPR / Cas9-PD-1-Puro, a plasmid pSPAX and pMD2.G are jointly transfected by 293T cells, and packaging of the recombinant virus Lenti-PD-1-Puro is completed. After T cells of peripheral blood of tumor patients are infected by the PD-1 recombinant virus, PD-1 on the T cells is successfully knocked out, the inhibitory state of the T cells of the tumor patients is relieved, the capacity of attacking tumor cells of the T cells embellished by the PD-1 recombinant virus is recovered accordingly, and the effect of immune cell treating is achieved.
Owner:安徽柯顿生物科技有限公司
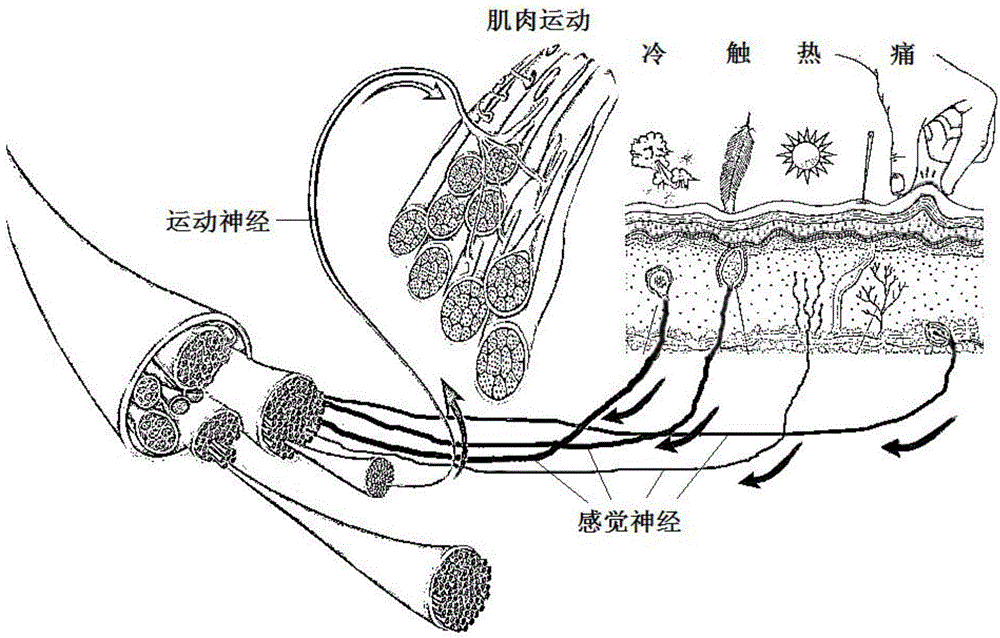
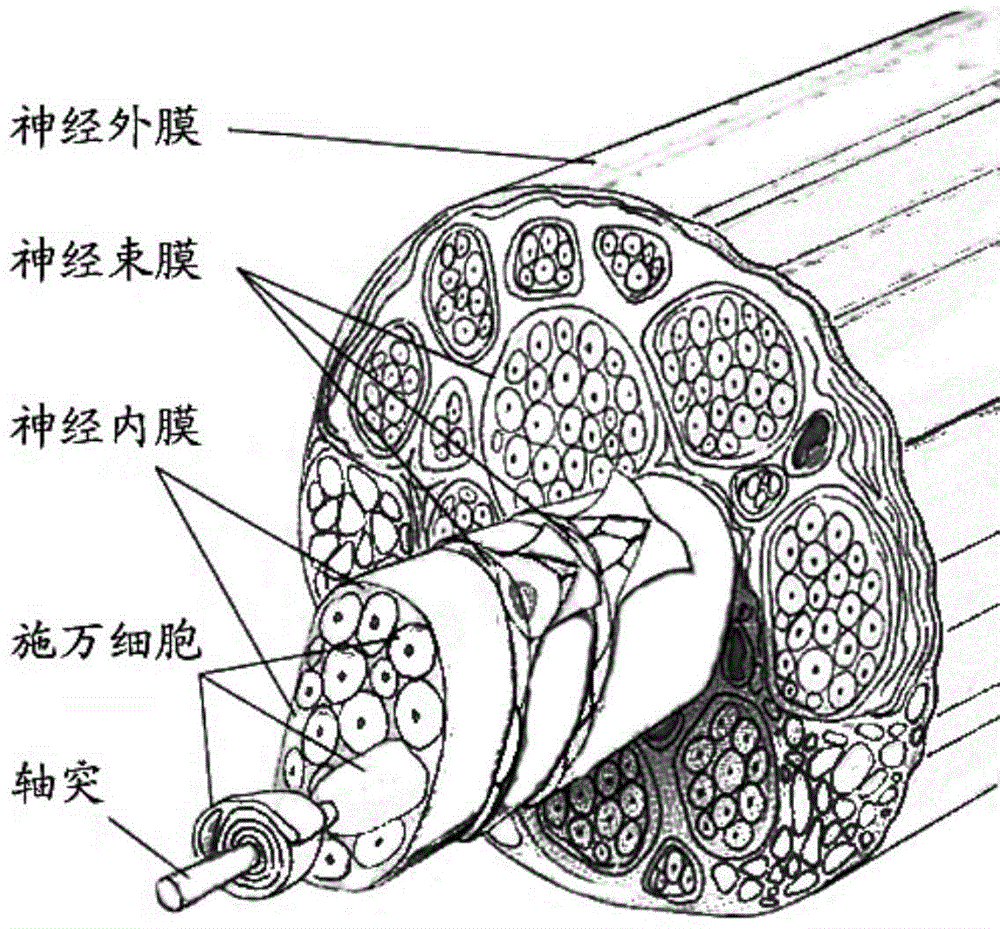
Tissue engineering nerve graft prepared by biological printing technology and preparation method thereof
The invention provides a tissue engineering nerve graft based on a biological printing technology and a preparation method thereof. The tissue engineering nerve graft comprises an outer tube and a tube inside scaffolds, wherein trophic factors and / or cells can cover the inner surface and the outer surface. A high-fidelity printing operation is performed according to the actual nerve shape demand by utilizing the biological printing technology, a polymer solution is printed into the specified nerve graft in a three-dimensional way by using an ink-jet printer by adjusting the sizes and the quantity of the jet nozzles of the ink-jet printer, the distances from the jet nozzles to a bottom layer and the pulse frequency of a supercharger and writing a control program of specific printing, and the specified nerve graft is applied to treatment of peripheral nerve defect and spinal cord injury.
Owner:南通大学技术转移中心有限公司
Acellular biological patch, preparation method and apparatus thereof
The invention relates to an acellular biological patch, a preparation method and an apparatus thereof. In the invention, small intestines of pigs are taken as raw materials, which are subjected to a series of decellularization treatment by means of an acid, hydrogen peroxide, a mixed solution of high salt and alkali so as to obtain the acellular biological patch. According to the preparation method, after treatment by the hydrogen peroxide, the mixed solution of high salt and alkali can be employed to conduct twice recycling treatment, thus reaching the effects of thorough decellularization and antigen removal. The employment of a transverse and longitudinal alternating laying way maintains uniformity of the patch's mechanical properties, the tensile strength, suture tear force, tear strength and other mechanical indexes can well meet clinical demands, thus facilitating surgical suture operation. The employed drying mode has no need of any adhesive and suture line for fixation, so that a natural three-dimensional support structure of the product is maintained, and after implantation into the human body, no strong inflammatory response and immunological rejection response can occur. The patch has no toxicity on cells, so that infection and adhesion cannot occur in a repair process. The acellular biological patch provided in the invention is suitable for repair of body tissue defects and soft tissue injuries, and also can be used in the bioengineering field to serve as the tissue for reinforcing repair of membranous defects and infected wounds and serve as a biological scaffold material, etc.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
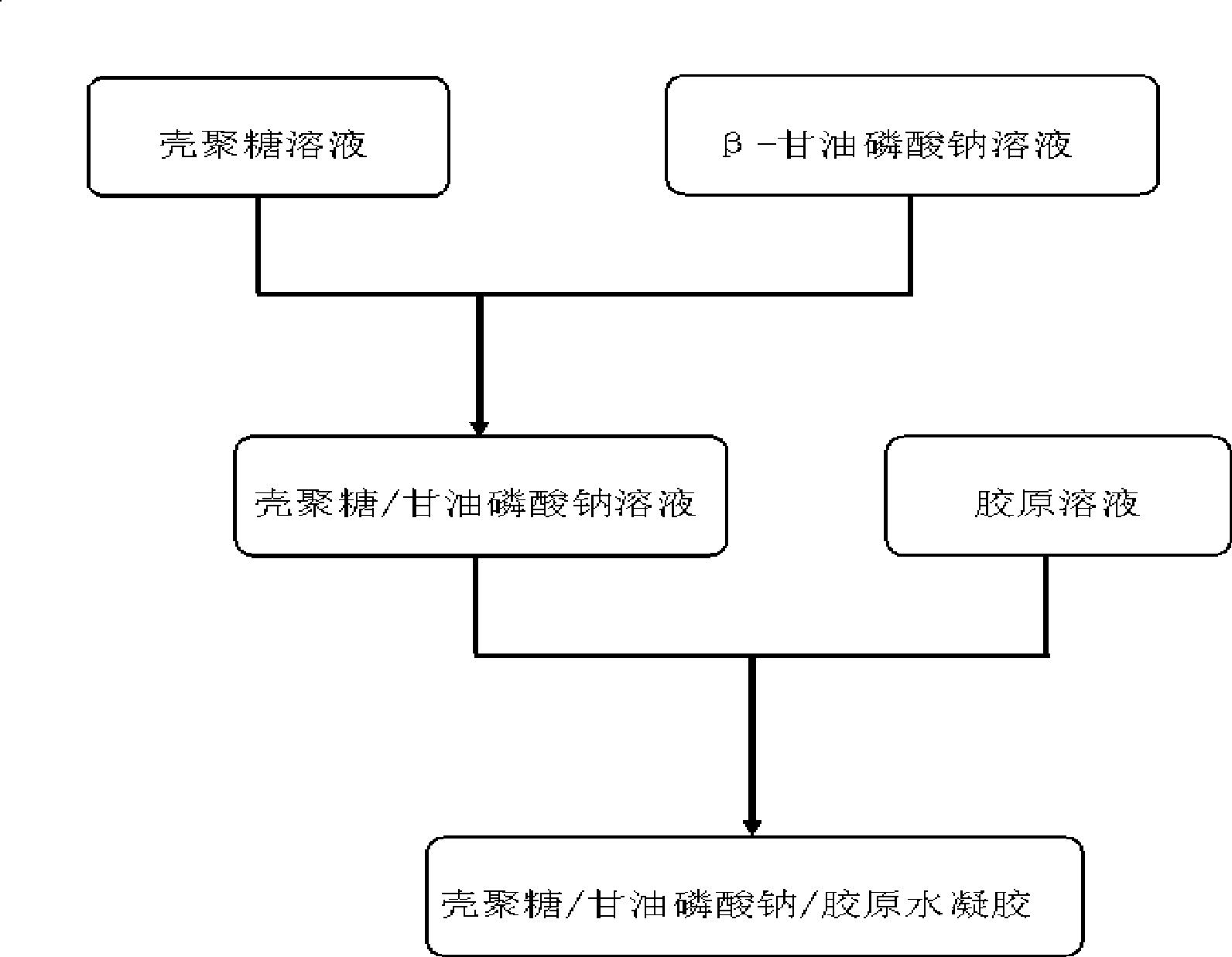
Method for preparing injectable chitosan/sodium glycerophosphate/collagen hydrogel
The invention discloses a method for preparing injectable chitosan / sodium glycero-phosphate / collagen hydrogel, belonging to the field of biotechnology and tissue engineering; the invention particularly relates to a technology for preparing temperature sensitive type injectable material. The method is characterized in that 2.2% (W / V) of chitosan solution is mixed with 50% (W / V) of beta-sodium glycero-phosphate solution at low temperature to prepare chitosan / sodium glycero-phosphate solution; then, the solution is mixed with collagen solution following a volume ratio of 1:10-10:1 at low temperature; the mixture is retained in the liquid state at room temperature( 8 DEG C) and is turned into gel at body temperature within 12min. The invention has the beneficial effects that the chitosan / sodium glycero-phosphate / collagen hydrogel can be maintained in the liquid state at low temperature when pH value is within physiological range (pH value ranges from 7.0 to 7.2); the mixture is turned into gel when the temperature is raised to body temperature (37 DEG C). As ideal cell growth substrate collagen is added, the hydrogel features fine biocompatibility.
Owner:DALIAN UNIV OF TECH
Method and application for inducing human umbilical cord mesenchyme stem cells to be differentiated into testicular interstitial cells
InactiveCN102174468AHigh differentiation efficiencyGood secretion effectSkeletal/connective tissue cellsViruses/bacteriophagesCorpus luteum graviditatisTesticular Interstitial Cells
The invention discloses a method and application for inducing human umbilical cord mesenchyme stem cells to be differentiated into testicular interstitial cells. The method comprises the following step of culturing human umbilical cord mesenchyme stem cells of patients suffering from adenovirus and carrying mice steroidogenic factor-1 genes in a DMEM-F12 culture solution containing 0.3-3ng / ml of luteinizing hormone, 200-800mu M of dibutyryl cyclic adenosine monophosphate, 5*10<-6>-5*10<-4>M of all-trans retinoic acid (ATRA), 10mU / ml of human chorionic gonadotropin and 2.4uM of adrenocorticotrophic hormone for a week. Induced by the method in the invention, the human umbilical cord mesenchyme stem cells can be differentiated into testicular interstitial cells in vitro and provides important sources of cells for treating testosterone shortage by the cell replacing method or the genetic method.
Owner:JINAN UNIVERSITY
Tissue engineering nerve graft and application thereof
The invention discloses a chitosan artificial nerve graft containing a neurotrophic factor and a preparation method thereof. In the chitosan artificial nerve graft, chitosan is processed into a nerve conduit with a porous structure and high tensile strength by performing processes such as weak acid dissolving, injection molding, molding, neutralization fixing, cleaning, freeze drying and the like under the condition of not adding any foaming agent or crosslinking agent. The nerve conduit contains cells or tissues which have treating effects and are included or distributed inside or on the surfaces of tubular body pores, including autologous bone marrow stem cells, autologous bone mesenchymal stem cells, schwann cells or dorsal root ganglion tissues or combinations thereof. A tissue engineering nerve can be used for repairing peripheral nerve defect and can be applied to repairing of spinal cord injury simultaneously.
Owner:NANTONG UNIVERSITY
Preparation method and device of duramater/spinal dural transplanting substitute
ActiveCN102727935ASimple Surface Functional StructureWidely sourced and cheapProsthesisAntigenDefect repair
The invention provides a preparation method of a duramater / spinal dural transplanting substitute which is obtained by repeated freezing and thawing of dural tissue, rolling and cracking of cells, crosslinking fixed protection, accellular antigen extraction, dense surface fibrosis modification, packaging and sterilization, and has the advantages of simple method, wide raw material sources, cheap raw materials, and low cost. The prepared dural substitute completely removes components of cells and other antigen components simultaneously when protecting dural tissue natural structure and properties, is good in biocompatibility, free of immune rejection, safe and reliable, good in mechanical performance, and easy in clinical operation, can meet the needs of defect repair, has the function of promoting tissue regeneration as a loose surface is beneficial to the tissue fluid adsorption, active factor enrichment, and growth of blood vessels and cells, and has the advantages of rapidness in fusion with a host, biodegradable absorption, and good repair effect. The animal test shows that the defect can be completely repaired without brain or spinal fluid leakage, or adhesion with brain tissue, and significant rejection is not found.
Owner:SHAANXI BIO REGENERATIVE MEDICINE CO LTD
3D bio-printing medical dressing and preparation method thereof
InactiveCN105031713ASimple manufacturing processEasy to operateAdditive manufacturing apparatusAbsorbent padsCell freeSide effect
The invention discloses a 3D bio-printing medical dressing and a preparation method thereof. The medical dressing takes cell-free collagen as a main component and is prepared by a rapid prototyping technology. According to the invention, the medical dressing prepared from a bio-material containing cell-free collagen has the advantages of good biocompatibility, no toxic or side effect and no immunological rejection; meanwhile, by using the rapid prototyping technology for preparing the dressing with a 3D structure, the preparation process is convenient and fast, the operation steps are simple, the geometric shape, pore diameter, porosity and pore distribution of the dressing can be accurately controlled according to wound sizes of different patients and the characteristics of the material, and thus personalized medical dressings with good air permeability and hydroscopic property for patients can be prepared.
Owner:SOUTH CHINA UNIV OF TECH
Umbilical cord mesenchymal stem cell injection and preparation method and application thereof
InactiveCN104922059ANo immune rejectionNon-tumorigenicPharmaceutical delivery mechanismUnknown materialsHydroxyethyl starchFiltration
The invention relates to umbilical cord mesenchymal stem cell injection and a preparation method and application thereof. The injection comprises umbilical cord mesenchymal stem cells and mixed solution; the mixed solution comprises balanced electrolyte solution, hydroxyethyl starch, adenosine disodium triphosphate-magnesium chloride, dimethyl sulfoxide and human serum albumin. The preparation method comprises the following steps: (1) fully and evenly mixing the balanced electrolyte solution, the hydroxyethyl starch and the human serum albumin, and performing filtration sterilization; (2) adding the umbilical cord mesenchymal stem cells; (3) adding the dimethyl sulfoxide; (4) performing split charging and frozen preservation to finish preparation; the invention also provides the application of the injection in the aspect of drugs for treating chronic ischemic heart diseases. The injection disclosed by the invention has a very good resuscitation effect after the frozen preservation, can be directly injected after being unfrozen and resuscitated, and has a remarkable effect on treatment on the chronic ischemic heart diseases.
Owner:北京青藤谷禧干细胞科技研究院有限公司
Organization engineering skin containing peripheral hematopoietic stem cells and method of preparing the same
InactiveCN101138654APromote proliferationPromote differentiationProsthesisMechanical wearTissue engineered skin
The present invention is a tissue engineering skin containing the peripheral blood stem cell and the preparation method. The present invention puts the peripheral blood stem cell and the skin fibroblast cell inside the biological frame. The active tissue engineering skin containing the peripheral blood stem cell is built by planting the epidermal cell on the surface. The tissue engineering skin is similar to the natural skin tissue in structure. Compared with the present skin replacing product, the present invention has properties of improving the blood supply in the wound and promoting the wound healing. The present invention can improve the elastic and toughness properties of the skin as well as the mechanical wear tolerance. The present invention can also reduce the scar proliferation and improve the healing quality. The present invention can improve the success rate of the artificial skin planting and can be effectively applied in repairing the skin defect, which is hard to heal.
Owner:陕西艾尔肤组织工程有限公司
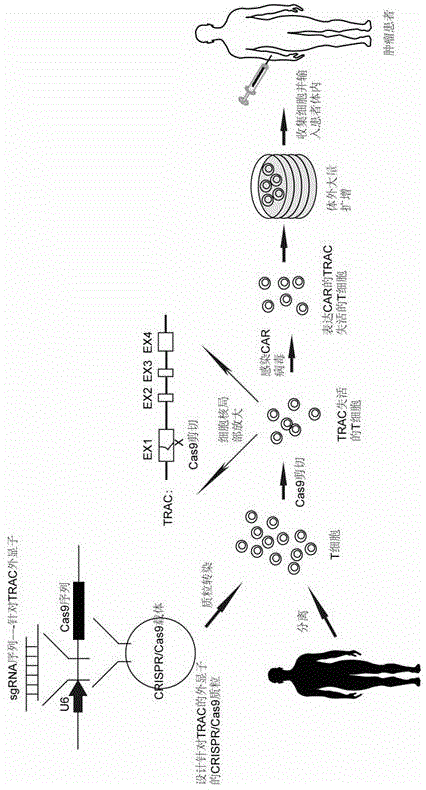
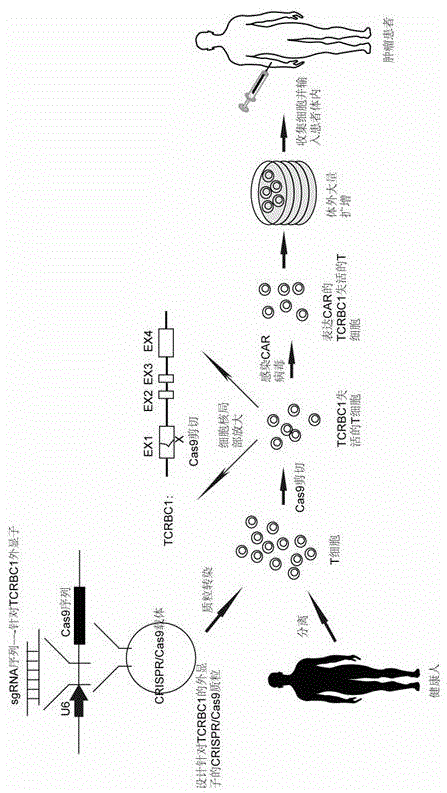
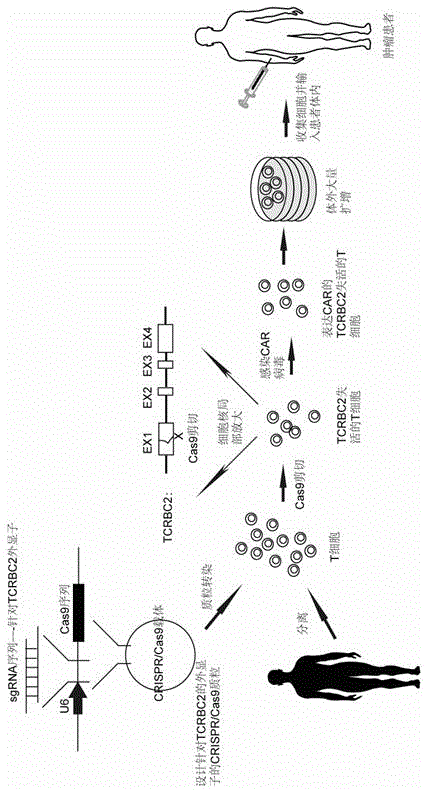
Chimeric antigen receptor T cell capable of conducting allograft and preparation method
InactiveCN105647871ANo immune rejectionFacilitate tumor treatmentCell receptors/surface-antigens/surface-determinantsForeign genetic material cellsChimeric antigen receptorWilms' tumor
The invention discloses a chimeric antigen receptor T cell capable of conducting allograft and a preparation method. The T cell is transformed through the gene fixed-point knockout technology, especially the CRISPR / Cas9 technology, gene knockout is conducted for the constant areas of chains alpha and beta of TCR of the T cell so that TCR of the T cell can be inactivated, and the T cell can conduct allograft without causing immunological rejection. The T cell with the inactivated TCR is combined with the CAR technology, and therefore the aim of treating specific tumors in a targeted mode through the T cell with the allograft source can be achieved. The chimeric antigen receptor T cell and the method overcome the defects that at present, T cells of patients are small in number, low in activity and long in culture time. High-quality and high-activity T cells in blood of healthy people can be used for transforming in advance, CART cells for various tumors are prepared, tumor patients do not need to wait once needing CART treatment, the precious time of treatment wait is shortened to the maximum extent.
Owner:BIOTOWNTEK CO LTD
Preparation and application of nerve tissue matrix derived tissue engineering scaffold material
InactiveCN102218160AKeep natural ingredientsGood biocompatibilityCatheterProsthesisFreeze-dryingDefect repair
The invention discloses preparation and application of a nerve tissue matrix derived tissue engineering scaffold material. A nerve tissue is taken as a raw material, matrix components (including nano-scale collagen microfilaments, fibronectin microfilaments and laminin microfilaments) favorable for nerve regeneration are extracted by means of medicine expansion, mechanical pulverization, enzymolysis treatment, dialysis collection and the like, and immunogenicity components (including Schwann cells, phospholipid and axons) not favorable for nerve regeneration are removed. The prepared nerve tissue matrix derived material can be further prepared into a three-dimensional porous oriented scaffold through oriented crystallization, freeze drying and crosslinking separately or by matching other polymer materials, or is prepared into a nano-scale film by an electrospinning technology, and the film is wound to form a nerve regeneration catheter. The tissue engineering scaffold or catheter prepared by the method is favorable for adhesion, proliferation and migration of seed cells, promotes nerve generation and can be used for never defect repair.
Owner:卢世璧
Preparation method and application thereof for cell-biological bracket compound based on biological print technology
InactiveCN103272288ABioprinting technology is simple and easyLow costSurgerySpinal cord lesionNerves regeneration
The invention provides a preparation method and the application thereof for a cell-biological bracket compound based on biological print technology. The cell-biological compound is formed by fibrous protein which is drawn out from the autoblood of a patient and processed by an ink-jet print technology. The surface and the interior of the cell-biological compound encompass one or more trophic factors and bone mesenchymal stem cells derived from self. According to the invention, the biological print technology is adopted, the appearance shape, cells and encompassing state model of the trophic factors can be designed according to requirements and practical situation, the cell-biological bracket compound is obtained through printing accurately, the fibrous protein and the bone mesenchymal stem cells are derived from self, so that the problem of immunological rejection is avoided, the trophic factors are cultivated in the bracket and are released slowly with the degradation of the bracket material, the cell-biological compound is applied to the spinal cord injured part to promote nerve regeneration and functional reconstruction on the injured part.
Owner:谢杨 +1
Biological surgical patch
ActiveCN1986007AInduction and promotion of regenerationConducive to regenerative repairSurgeryProsthesisAntigenRegeneration tissue
The present invention discloses biological surgical patch and its preparation process. The biological surgical patch includes one base material of animal membrane material, which is cross-linking fixed with non-aldehyde fixing agent and treated to eliminate antigen. The biological surgical patch made of natural biological material with collagen as main component may be degraded in the degradation speed synchronizing with the growth of the regenerating tissue, and the degraded products are amino acids or polypeptide capable of being absorbed by organism for the regenerative repair of the defected tissue. The present invention has no immunological rejection reaction, high biocompatibility, capacity of inducing tissue regeneration and good mechanical compliance, and can meet the requirement for repairing tissue.
Owner:GRANDHOPE BIOTECH CO LTD
Preparation method of tissue engineering porous extracellular matrix scaffold
ActiveCN105561398AHigh mechanical strengthSolve the problem of holesProsthesisAcellular scaffoldPolymer scaffold
The invention provides a preparation method of a tissue engineering porous extracellular matrix scaffold. The method is characterized in that a nano or micron polymer scaffold is implanted under the skin of or in the abdomen of a host, the polymer scaffold is used as a template to prepare the tissue engineering porous extracellular matrix scaffold with a controllable pore structure and free of immunogenicity after in-vivo implantation by using a host immunoprotection mechanism. The preparation method has the advantages that the problem of hole forming of extracellular matrix scaffold materials is solved; the porous scaffold is animal-source, is used for repairing self-injured tissue or organs, and is free of immunological rejection; since self-repair has no complex decellularization step, the scaffold has significantly better mechanical strength than a decelluarized scaffold material; the extracellular matrix scaffold may be further decelluarized for repairing foreign tissue or organs and has a promising application prospect in the field of organ repairing.
Owner:NANKAI UNIV
Transplanting material of fat granule tissues compounded with SVFs (Stromal Vascular Fractions) and PRFs (Platelet-Rich Fibrins) as well as preparation method and application thereof
The invention belongs to the technical fields of tissue engineering and biological materials, in particular to a transplanting material of fat granule tissues compounded with SVFs (Stromal Vascular Fractions) and PRF (Platelet-Rich Fibrins) as well as a preparation method and application thereof. The transplanting material comprises fat granules, SVFs and PRF membrane granules. The preparation method comprises the following steps of: extracting fat granules from a patient by adopting liposuction, additionally extracting SVFs obtained by fat granule digestion from the same patient and extracting PRF membrane granules obtained by blood centrifuging from the same patient; mixing the SVFs with the PRF membrane granules; incubating at the temperature 37 DEG C for 10 minutes; and mixing the fatgranules, the SVFs and the PRF membrane granules to obtain the transplanting material of fat granule tissues compounded with the SVFs and the PRFs. The material can be applied to soft tissue molding and soft tissue defect repairing; by adopting the material, the absorption of transplanted fat tissues is effectively reduced, and the fat transplanting effect is improved.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Polypeptide, production method and uses thereof
ActiveCN109293783APromote cell adhesionAchieve cell adhesion activityConnective tissue peptidesBacteriaBiotechnologyCell adhesion
The present invention relates to a polypeptide, a production method and uses thereof, wherein the polypeptide comprises an N-terminal region and a C-terminal region, and has remarkable cell adhesion effect.
Owner:SHANXI JINBO BIO PHARMA CO LTD
Functional tissue engineering material for nerve repair, preparation method and use thereof
The present invention provides a functional tissue engineering material for nerve repair. The functional tissue engineering material comprises human amnion, a neurotrophic factor specifically bound with the collagen, and an inhibitor fixedly bound with calpain. The present invention further provides a preparation method for the functional tissue engineering material, wherein neurotrophic factor fusion protein specifically bound with the collagen binding domain and an inhibitor for cross-linking calpain are constructed to form the functional tissue engineering material for guiding nerve repair, such that the human amnion can be specifically bound with the neurotrophic factor so as to effectively promote nerve repair, the regeneration nerve forms correct synaptic connections, the nerve re-injury effect of calpain can be eliminated, and the material can be used for targeting treatment of central nerve injury diseases and neural function repair.
Owner:PLA NAVY GENERAL HOSIPTAL
Injectable chitosan composite hydrogel capable of promoting myocardium repair and preparation method of injectable chitosan composite hydrogel
The invention discloses injectable chitosan / keratin composite hydrogel capable of promoting myocardium repair and a preparation method of the hydrogel. The hydrogel consists of four solutions, namely chitosan, sodium beta-glycerophosphate, genipin and keratin according to a volume ratio of 10: (1-5): (1-3): (0.1-0.5). The preparation method comprises the following steps: 1) extracting keratin from human fairs by using peracetic acid and Tris alkaline; 2) adjusting the pH value of a water-soluble chitosan solution by using sodium beta-glycerophosphate so as to be close to a microenvironment in a human body; 3) introducing genipin as a crosslinking agent, and controlling the gelling time of a hydrogel solution by changing the use amount of the genipin so as to achieve the purpose that the mixed solution can be rapidly gelled in situ after being injected into an organism. Experiments prove that the composite hydrogel has favorable biocompatibility and has the capability of remarkably promoting the growth of myocardial cells; the hydrogel is wide in sources of materials and low in cost and has a broad application prospect in the field of tissue engineering repair.
Owner:SOUTHEAST UNIV
Stem cell repairing material as well as preparation method and application thereof
InactiveCN102205146ATraumaLittle painMammal material medical ingredientsDermatological disorderMesenchymal stem cellAutologous Fat Graft
The invention relates to a stem cell repairing material as well as a preparation method and application thereof. The stem cell repairing material comprises a stem cell and a cell carrier, wherein the stem cell is an autologous adipose-derived mesenchymal stem cell (ADSC) with a concentration of 105-108 / ml, and the cell carrier is autoserum, normal saline (0.9%) or a glucose injection (5%). In the invention, adopted seed cells are prepared through separating and purifying autologous adipose tissues of patients, thereby avoiding the ethical disputes and the immunological rejection. The material drawing of the adipose tissues can be performed by using an instrument suction method, which is simple in operation and can bring less traumas and pains to the patients. The cell carrier used in the invention is the autoserum, normal saline (0.9%) or glucose injection (5%), therefore, the cell carrier mixed with mesenchymal stem cells and other ingredients can be injected into the patents by virtue of syringes. By using the stem cell repairing material provided by the invention, the operation process is simple, no scar is left, and the wounds and pains for the patients are avoided, therefore, the stem cell repairing material can be easily accepted by the patients.
Owner:王影
Recombinant humanized III-type collagen and preparation method thereof
PendingCN112194720AHigh expressionSuitable for large-scale scale-upConnective tissue peptidesMicroorganism based processesBase JEngineering
The invention provides a recombinant humanized III-type collagen. The recombinant humanized III-type collagen has a triple helix structure, and has an amino acid sequence reducing a humanized III-typecollagen alpha-1 chain and including 1068 amino acids. The invention further provides a preparation method of the recombinant humanized III-type collagen, wherein the prolyl hydroxylase of PBCV-1 includes 209 amino acids, and the base sequence is optimized according to the codons of saccharomyces cerevisiae. Compared with the prior art, the preparation method has the advantages that the triple helix is formed in vitro from collagen chains by utilizing the prolyl hydroxylase; and the saccharomyces cerevisiae expression system adopted by the invention is applicable to large-scale amplificationwithout endotoxin or later removal, the preparation cost is low, and the protein expression quantity is further improved. The recombinant humanized III-type collagen is excellent in stability, the amino acid composition of the recombinant humanized III-type collagen is almost completely the same as that of the alpha-1 chain of a natural collagen, and no immunological rejection can be generated when the recombinant humanized III-type collagen is applied to a human body. The recombinant humanized III-type collagen is good in biocompatibility and can be widely applied to surgical treatment and cosmetic medical industry.
Owner:叶华
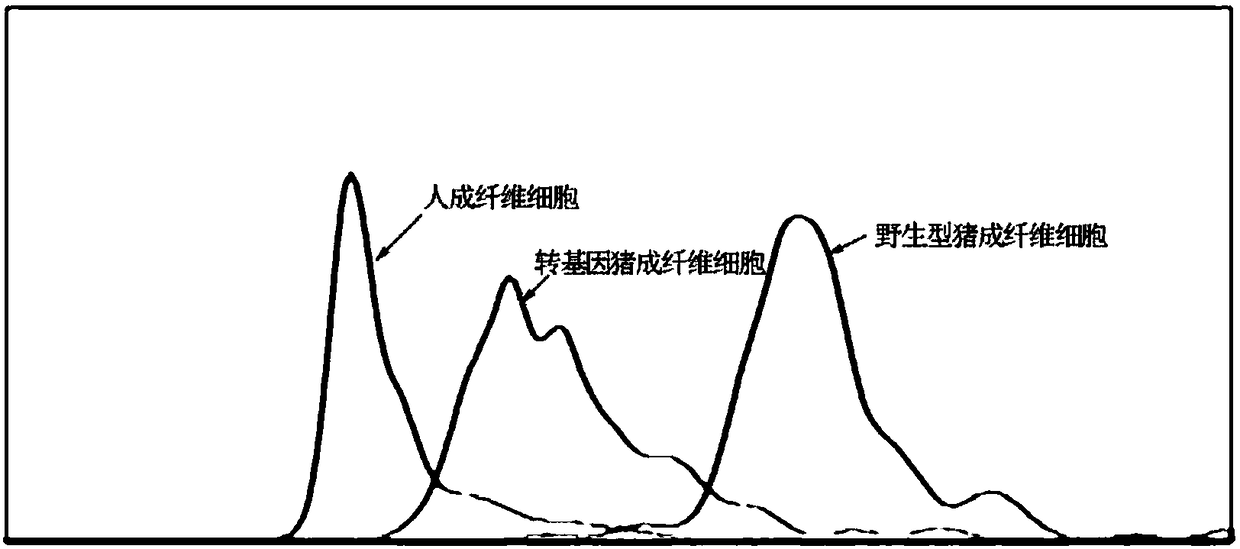
Culture method of transgenic swine, and applications thereof
ActiveCN108486152AMake sure to cloneClone realityGenetically modified cellsSkeletal/connective tissue cellsEnzymeSomatic cell
The invention discloses a culture method of transgenic swine. The culture method at least comprises following steps: swine primary somatic cells are introduced into messenger RNA coded cell reprogramming genes and / or miR302 / 367 clusters for inductive production of RNA inductive multipotential stem cells; under the effect of Cas9-gRNA and CRE homologous recombinase, swine toxic genes are knock out,and knock of a plurality of human gene segments is carried out, human derived vWF gene is used for replacing swine endogenous vWF gene, and obtaining PERV-virus-inactivated human-immunological-rejection-free coagulation-incompatibility-free genes; clean target cell lines without off-target-effect are selected, and are culture into swine somatic cells; somatic cell nucleus transfer technology is adopted to treat swine somatic cells so as to obtain transgenic swine. The transgenic swine possesses following advantages, permanent PERV virus expression is inhibited permanently, human immunologicalrejection is avoided, and blood coagulation compatibility is achieved, and can be taken as human heterogeneous organ, tissue, and cell donors.
Owner:GENEO MEDICINE CO LTD
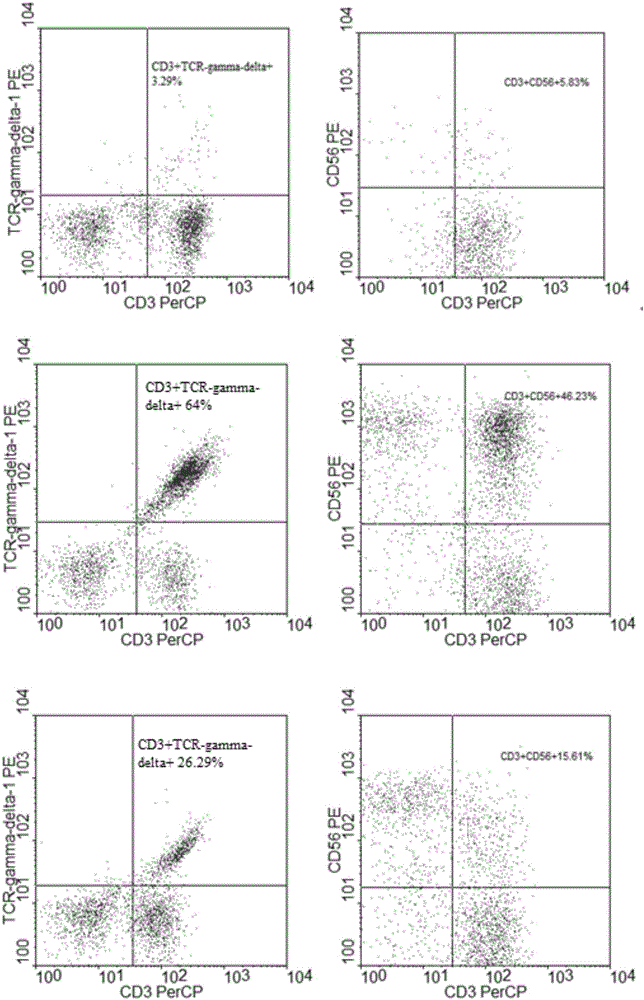
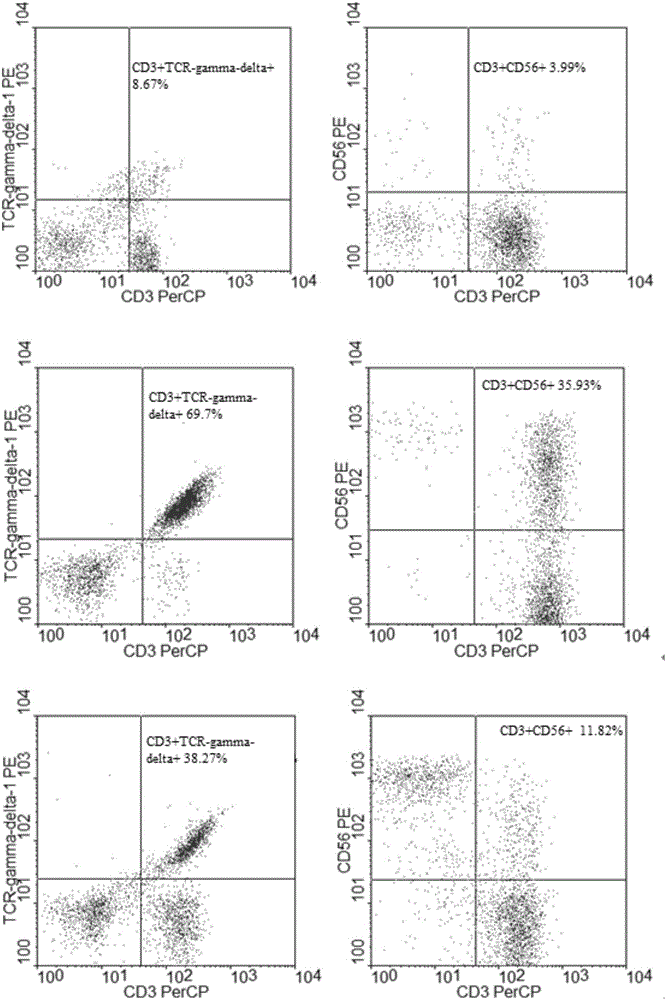
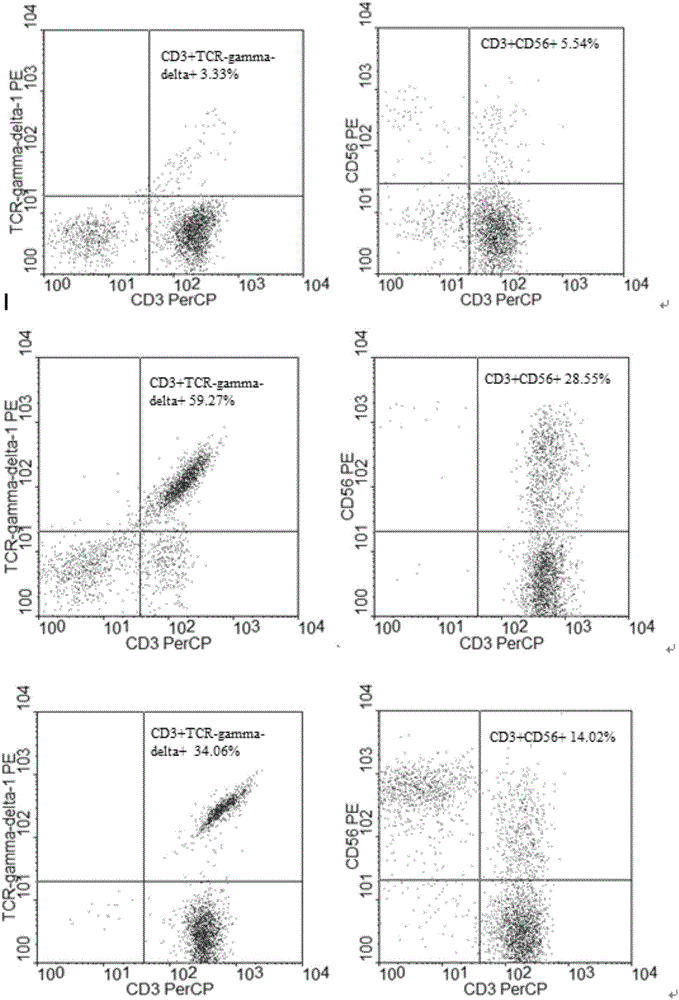
Method for jointly preparing CAR-VGamma9VDelta2T cells and CAR-NKT cells
ActiveCN106399242ANo immune rejectionEfficient killingCulture processBlood/immune system cellsPeripheral blood mononuclear cellCell culture media
The invention discloses a method for jointly preparing CAR-VGamma9VDelta2T cells and CAR-NKT cells, comprising the steps of 1) adding peripheral blood mononuclear cells to a cell medium, pre-activating VGamma9VDelta2T cells and NKT cells therein, and amplifying the VGamma9VDelta2T cells and NKT cells by selective in-vitro activation; 2) transducing the mixed cells of step 1) by using a lentiviral vector carrying chimeric antigen receptor's gene sequence. It is possible to carry out standard and batch preparation herein by using GammaDeltaT of a healthy donor; the purities of the VGamma9VDelta2T cells and NKT cells in the application can reach 60% and 30% and above respectively without purification, and therefore pre-purification can be omitted; in-vivo activation proliferation level and existence time of CAR-VGamma9VDelta2T cells and CAR-NKT cells jointly prepared by using the method can be controlled through clinical medication.
Owner:BEIJING DOINGTIMES INST OF TRANSLATIONAL MEDICINE
Neural stem cell injection for treating senile dementia and Parkinson's disease
ActiveCN1966080ANo immune rejectionResume deliveryNervous disorderPharmaceutical active ingredientsTransfer cellSingle cell suspension
The invention relates to a nerve stem cell injection made from retina pigment epithelial cell, to treat paralysis agitans, wherein said injection at least comprises 3*106 nerve stem cells of human hRPE, while said nerve stem cells are cultivated from retina pigment epithelial cells. And its production comprises that: (1), separating and cultivating cells, that separating the hRPE cells, mixing uniformly to prepare single cell suspension; (2), hRPE cell cultivation and generation that digests original cultivation liquid, ending digestion, blowing and beating to form cell suspension, eccentrically filtering and removing the upper clear liquid, adding cultivating substrate, to disperse and form cell suspension, filtering with cell screen, transferring cell into cultivating bottle, generating to at least fifth generation; (3), adding common salt into hRPE nerve stem cell, to prepare cell suspension injection.
Owner:北京拓华伟业生物科技有限公司
Fat filler and preparation method thereof
ActiveCN106421920AEasy to transplantImprove adhesionTissue regenerationProsthesisVascular endotheliumFibrosis
The invention discloses an autologous fat filler and a preparation method thereof, wherein the autologous fat filler is constructed by simulating a tissue engineering method. The autologous fat filler comprises the following ingredients of a cytoskeleton, a cellular constituent, a growth factor and a scar resistant factor at the volume ratio of (109-115):(15-30):4:0.5. The effective ingredients are combined, the cytoskeleton part provides a skeleton made of a plant cell ingredient, an injectable gel is favorable for the adhesion of a cell, a washing fat occupies most of a filling volume, a stem cell and an SVF (Stromal Vascular Fraction) component are differentiated into an adipose cell and a vascular endothelial cell, and various excreted factors are good for maintaining a transplanting microenvironment and generating a blood vessel. According to the provided autologous fat filler and the preparation method thereof, the stability of the transplanting fat is effectively improved, the absorption of the transplanted fat is reduced, the untoward effect of fibrosis of a transplant is reduced, and the development of an autologous fat face filling plasty is promoted.
Owner:沈阳细胞治疗工程技术研发中心有限公司
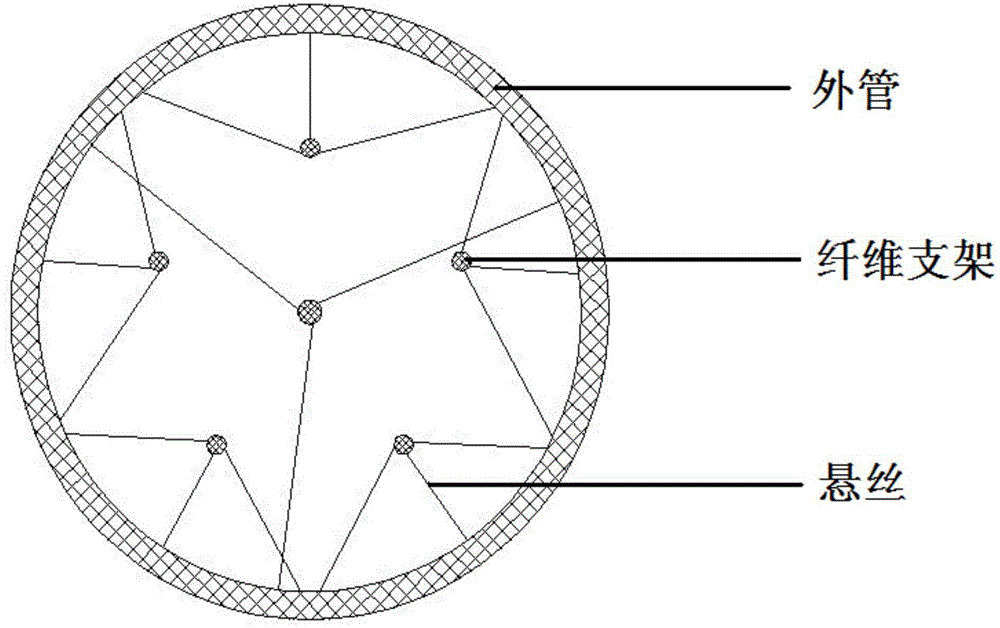
Tissue engineering nerve graft with suspension fiber scaffold and preparation method thereof
The invention discloses a tissue engineering nerve graft with a suspension fiber scaffold. The nerve graft comprises a conduit and tube fiber scaffolds. The nerve graft is characterized in that a plurality of suspensions are arranged on each fiber scaffold along the fiber length direction, and are connected with the inner wall of the conduit; and the fiber scaffolds are distributed and suspended in the conduit by virtue of the suspensions. By utilizing bio-printing technology, built-in microfilaments of a tissue engineering nerve graft can be stably and uniformly distributed in the conduit by utilizing the supporting force of the suspensions, and each fiber scaffold has an independent space to guarantee that nerve cells can directionally grow along the fiber scaffolds and cannot have error bridging, so that the success rate of operations can be greatly improved.
Owner:NANTONG UNIVERSITY
Preparation method for degradable three dimensional fiber scaffold capable of promoting repair of bone defects
The invention provides a preparation method for a degradable three dimensional fiber scaffold capable of promoting the repair of bone defects bone defect, and relates to a preparation method for a nano fiber scaffold material based on an electrospinning technique. The preparation method combines a biological material of polyhydroxybutyrate-hydroxyvalerate copolyester (PHBV) which is degradable, has no immunogenicity but cannot induce osteogenesis, polyoxyethylene (PEO) which has good water solubility and can promote the scaffold to be degraded rapidly and hydroxyapatite (nHA) which has bone conductive capacity but need a certain vector. An electrospinning fiber membrane with nano-scale diameter is obtained via an electrostatic spinning method; and finally a multilayer rod-shaped degradable three-dimensional scaffold material can be obtained via cutting and folding. Animal experiments demonstrate that the scaffold has good biocompatibility and appropriate degradable speed, and can realize the repair of large-size bone defects.
Owner:SOUTHEAST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com