Polypeptide, production method and uses thereof
A sequence and region technology, applied in the field of genetic engineering, can solve the problems of long production cycle, loss of biological activity of collagen, and high cost, and achieve good hydrophilicity and stability, good cell adhesion effect, and low production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1: Construction and expression of recombinant human collagen polypeptide
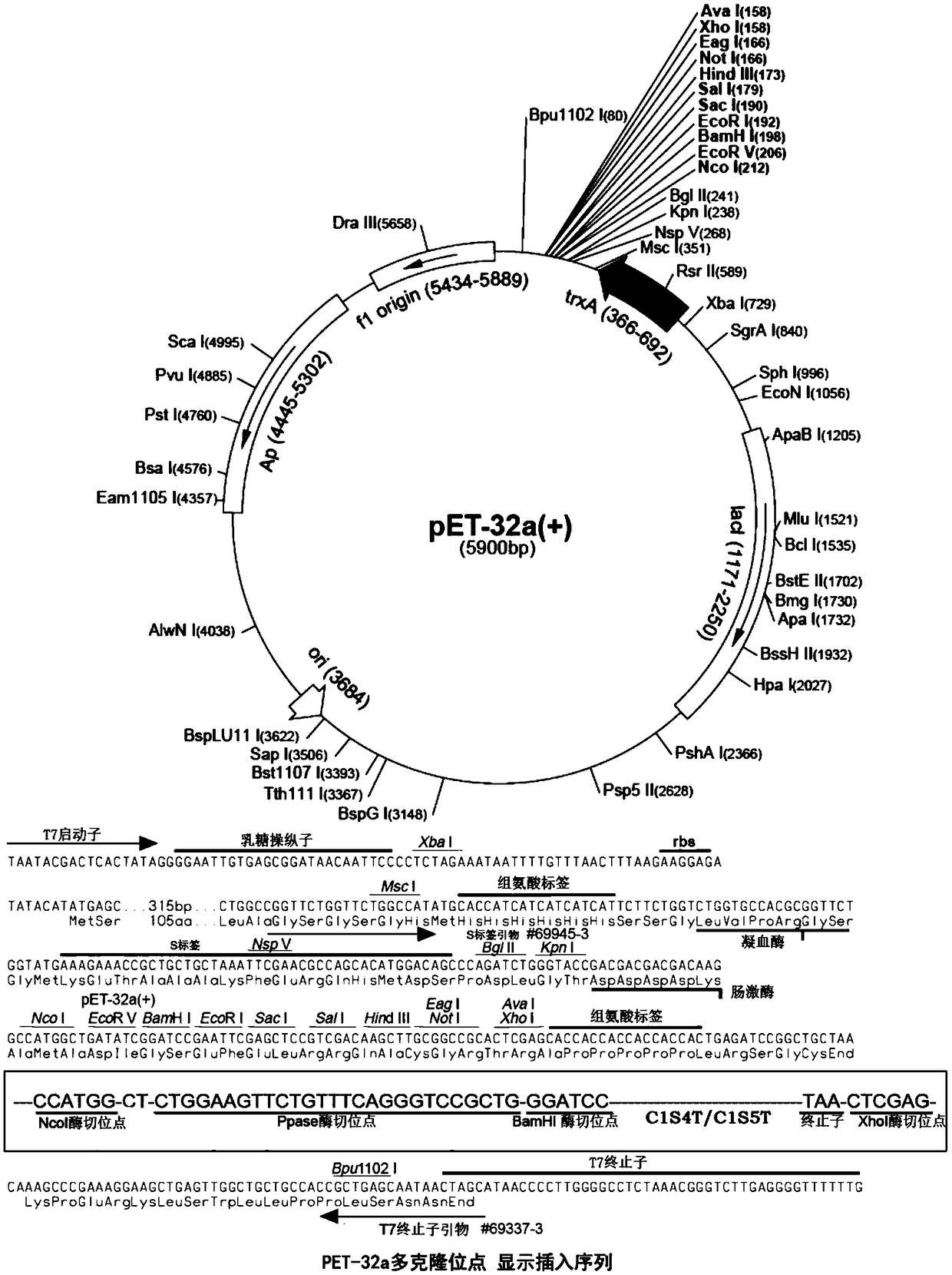
[0069] Construction and expression of C1S4T gene expression vector
[0070] 1. The full-length gene sequence of human collagen C1S4T used in Example 1 is shown in SEQ ID No.7. This sequence has been codon optimized for E. coli codons.
[0071]2. The full length of the C1S4T gene is 747bp. According to the optimized C1S4T codon gene sequence SEQ ID No.7, Shanghai Huajin Biotechnology Co., Ltd. was entrusted to synthesize the gene fragment, and the synthesized C1S4T gene fragment was passed through BamHI (NEB Company Product number: R0136L) and Xho I (NEB company, product number: R0146L) restriction sites were inserted into PET32a expression vector. The successfully constructed expression plasmid was transformed into Escherichia coli competent cell BL21(DE3) (Merck Company). The specific process is as follows: 1: Take 1 μl of the plasmid in 100 μl of Escherichia coli competent cells BL...
Embodiment 2C1
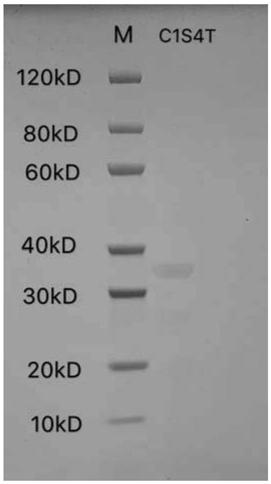
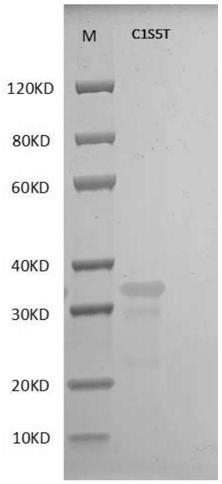
[0085] Activity detection of embodiment 2C1S4T and C1S5T protein
[0086] Collagen activity detection method can refer to the literature Juming Yao, Satoshi Yanagisawa, Tetsuo Asakura, Design, Expression and Characterization of Collagen-Like Proteins Based on the Cell Adhesive and Crosslinking Sequences Derived from Native Collagens, J Biochem.136, 643-649 (2004). The specific implementation method is as follows:
[0087] 1. Use the ultraviolet absorption method to detect the concentration of the protein samples to be tested, including control human collagen (Sigma, C7774), C1S4T and C1S5T protein samples. Specifically, the ultraviolet absorption of samples at 215nm and 225nm was measured respectively, and the protein concentration was calculated by using the empirical formula C(μg / mL)=144X(A215-A225), and it should be detected under the condition of A215<1.5. The principle of this method is to measure the characteristic absorption of peptide bonds under far-ultraviolet light...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com