Recombinant humanized III-type collagen and preparation method thereof
A technology of human collagen and production method, which is applied in the field of recombinant type III human collagen and its production, and can solve problems such as inability to perform biological functions, human immune rejection, and hidden dangers of viruses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Construction of co-expression vector of human collagen type III gene and PBCV-1 proline hydroxylase:
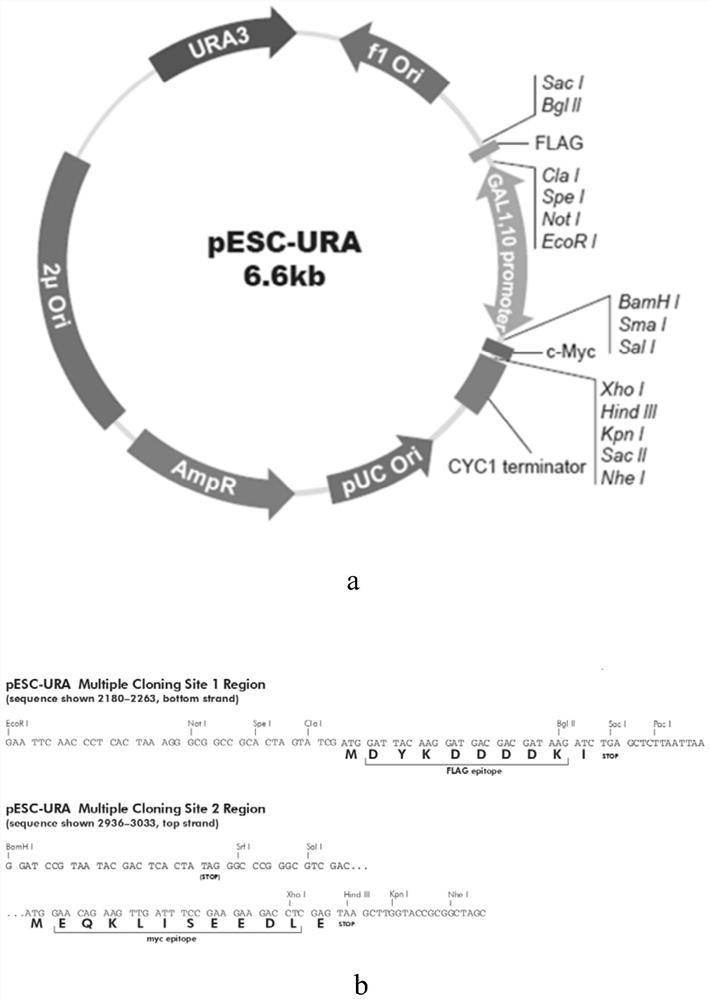
[0041]Add BglII and SacI restriction sites to the 5' and 3' ends of the optimized human collagen type III gene, synthesize the gene, transform and extract the plasmid vector where a large number of genes are located, digest with BglII and SacI, and agarose gel Recovery and purification. The pESC-URA plasmid vector was digested and purified by the same method. The human collagen type III gene fragment and the pESC-URA plasmid vector fragment were ligated with T4 DNA ligase overnight at 16°C, transformed into Escherichia coli DH5α, the clones were screened on the ampicillin LB plate, and the plasmid was extracted and identified by double enzyme digestion (ClaI— SacI), select the successful single clone, expand the culture and extract the plasmid as a vector, digest it with BamHI and SalI, recover and purify it on agarose gel. Add BamHI and SalI restriction sites to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com