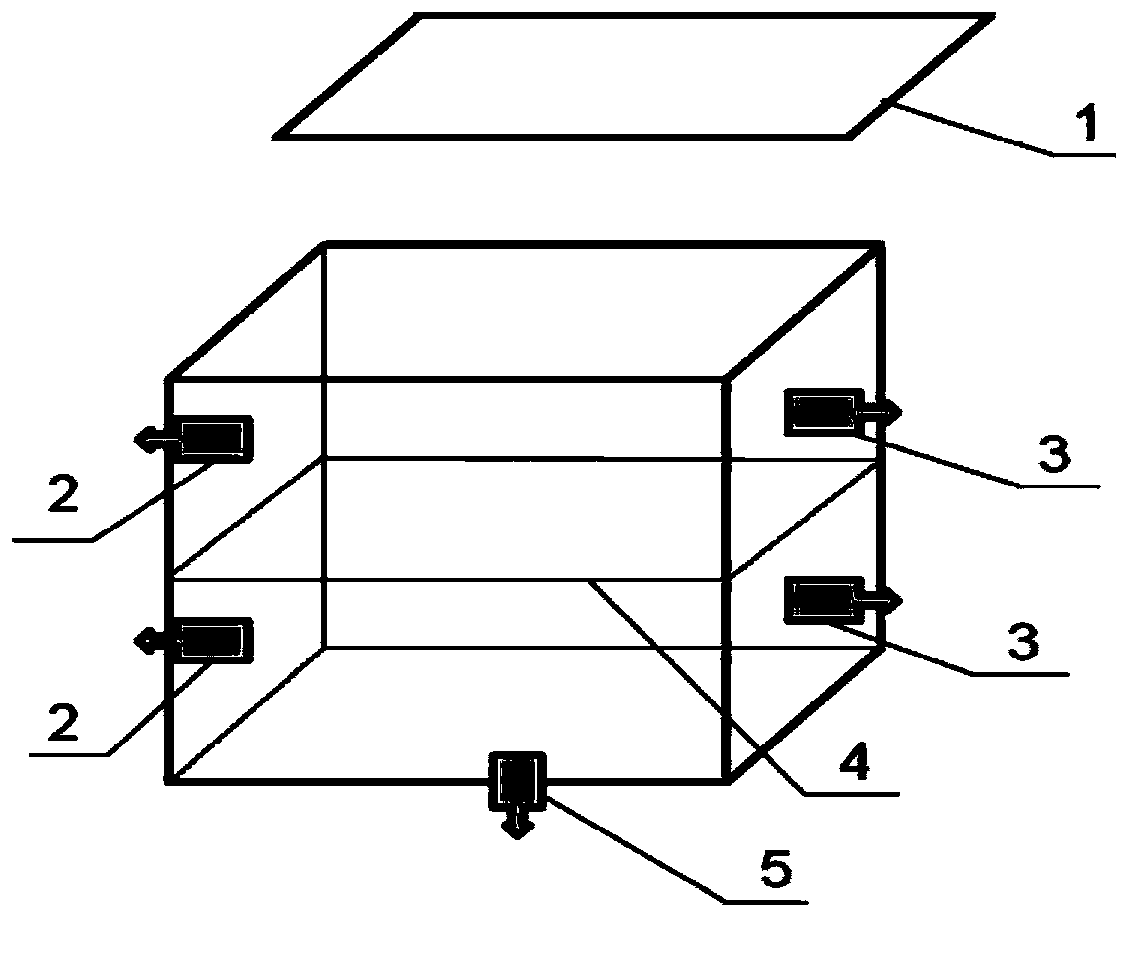
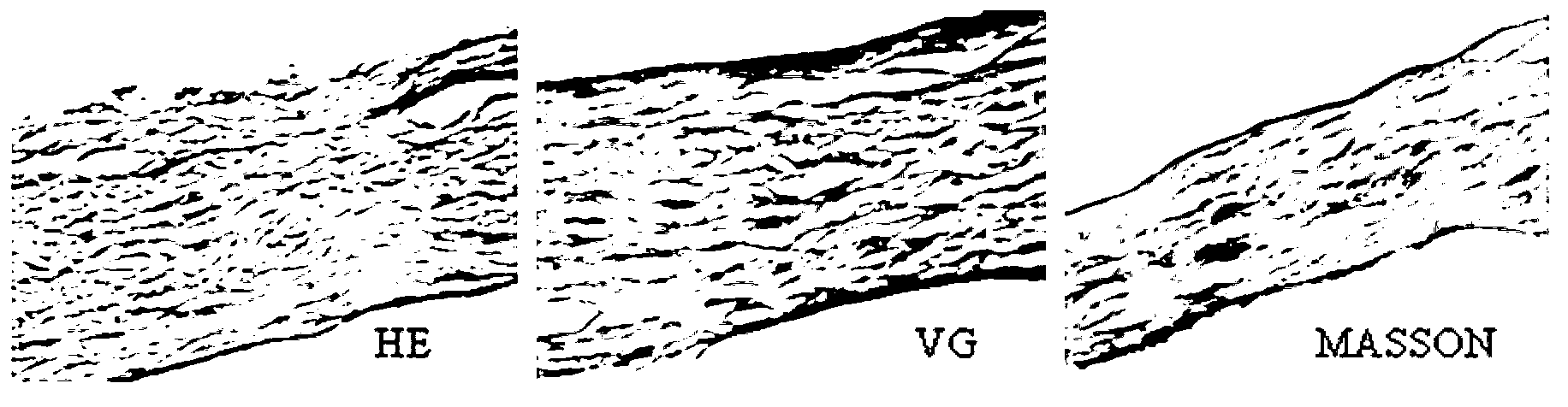
Acellular biological patch, preparation method and apparatus thereof
A technology of biological patch and preparation device, which is applied in the field of medical biomaterials in tissue engineering, can solve the problems of incomplete decellularization and antigen removal, inflammatory reaction, immune rejection, etc., so as to overcome uneven mechanical strength and avoid immune Rejection, the effect that is conducive to industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1, One of the preparation method and device of decellularized biological patch
[0057] The preparation method and steps of the present embodiment are as follows:
[0058] Step 1. Pretreatment: Wash the pig small intestine that has been cut open with the mucous membrane layer and lymph nodes removed with purified water until there is no dirt in the intestine, then soak it in aqueous hydrochloric acid solution with a volume concentration of 2.0% for 2.5 hours, and then use purified Wash with water until the pH value is 6.0-8.0.
[0059] Step 2. Disinfection treatment: Place the pig small intestine treated in step 1 in a hydrogen peroxide solution with a volume concentration of 15%, soak for 0.5 hours, wash with purified water for 3 times, scrape off the muscular layer and serosa layer with a blade, and retain the mucous membrane Lower layer: Soak the small intestinal submucosa in 70% ethanol solution for 1 hour, rinse with purified water until odorless.
[0...
Embodiment 2
[0066] Embodiment 2, the preparation method and device of decellularized biological patch 2
[0067] The preparation method and steps of the present embodiment are as follows:
[0068] Step 1. Pretreatment: Wash the pig small intestine that has been cut open with the mucous membrane layer and lymph nodes removed with purified water until there is no dirt in the intestine, then soak it in 3.0% lactic acid aqueous solution for 1.5 hours, and then use purified Wash with water until the pH value is 6.0-8.0.
[0069] Step 2. Disinfection treatment: place the pig small intestine treated in step 1 in a hydrogen peroxide solution with a volume concentration of 1%, soak for 2.5 hours, wash with purified water twice, scrape off the muscular layer and serosa layer with bamboo slices, and keep Submucosa: After soaking the submucosa of the small intestine in isopropanol solution for 3 hours, rinse it with purified water until it is odorless.
[0070] Step 3. Decellularization and antigen...
Embodiment 3
[0074] Embodiment 3, the third preparation method and device of decellularized biological patch
[0075] The preparation method and steps of the present embodiment are as follows:
[0076] Step 1, pre-treatment: the small intestine of the pig that has been removed from the mucous membrane layer and lymph nodes is washed with purified water until there is no dirt in the intestine, and then soaked in a peracetic acid solution with a volume concentration of 1.5% for 1.0 hour, and then Wash with purified water to pH 6.0-8.0.
[0077] Step 2. Disinfection treatment: place the pig small intestine treated in step 1 in 3% hydrogen peroxide solution, soak for 1.5 hours, wash with purified water for 3 times, scrape off the muscular layer and serosal layer with a stainless steel plate, and keep Submucosa: Soak the submucosa of the small intestine in 80% ethanol solution for 2 hours, rinse with purified water until odorless.
[0078] Step 3, decellularization and antigen removal: using ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com