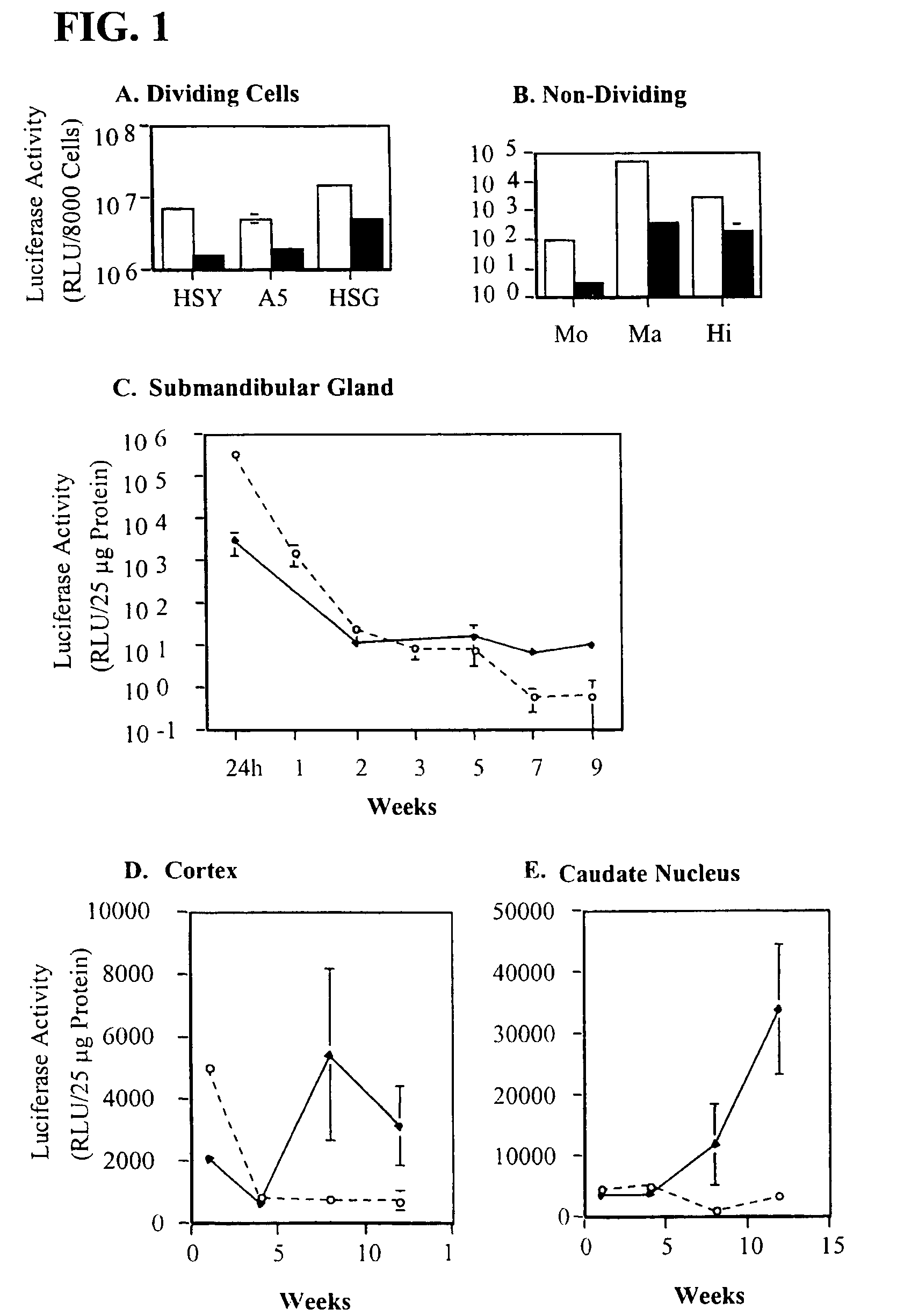
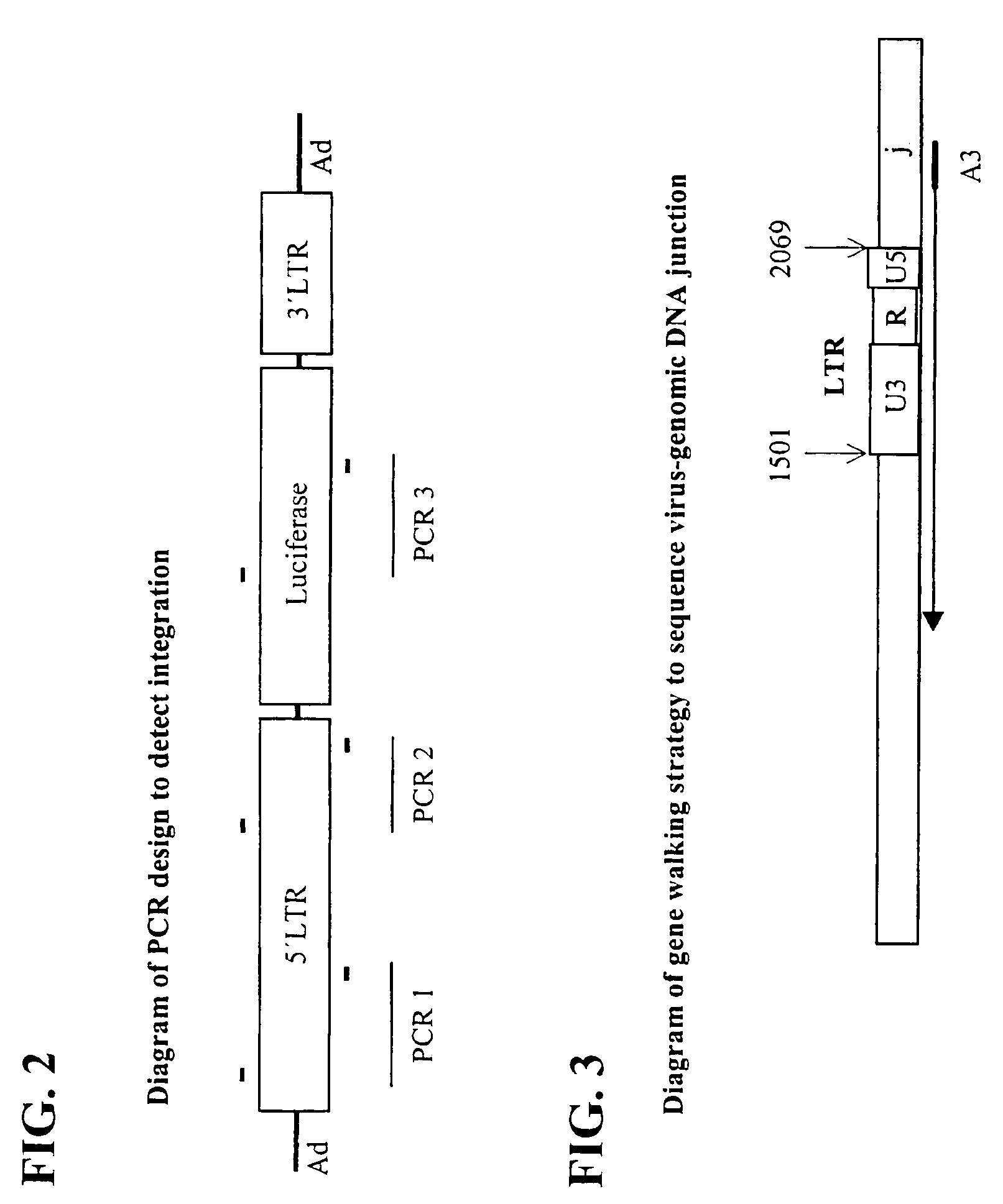
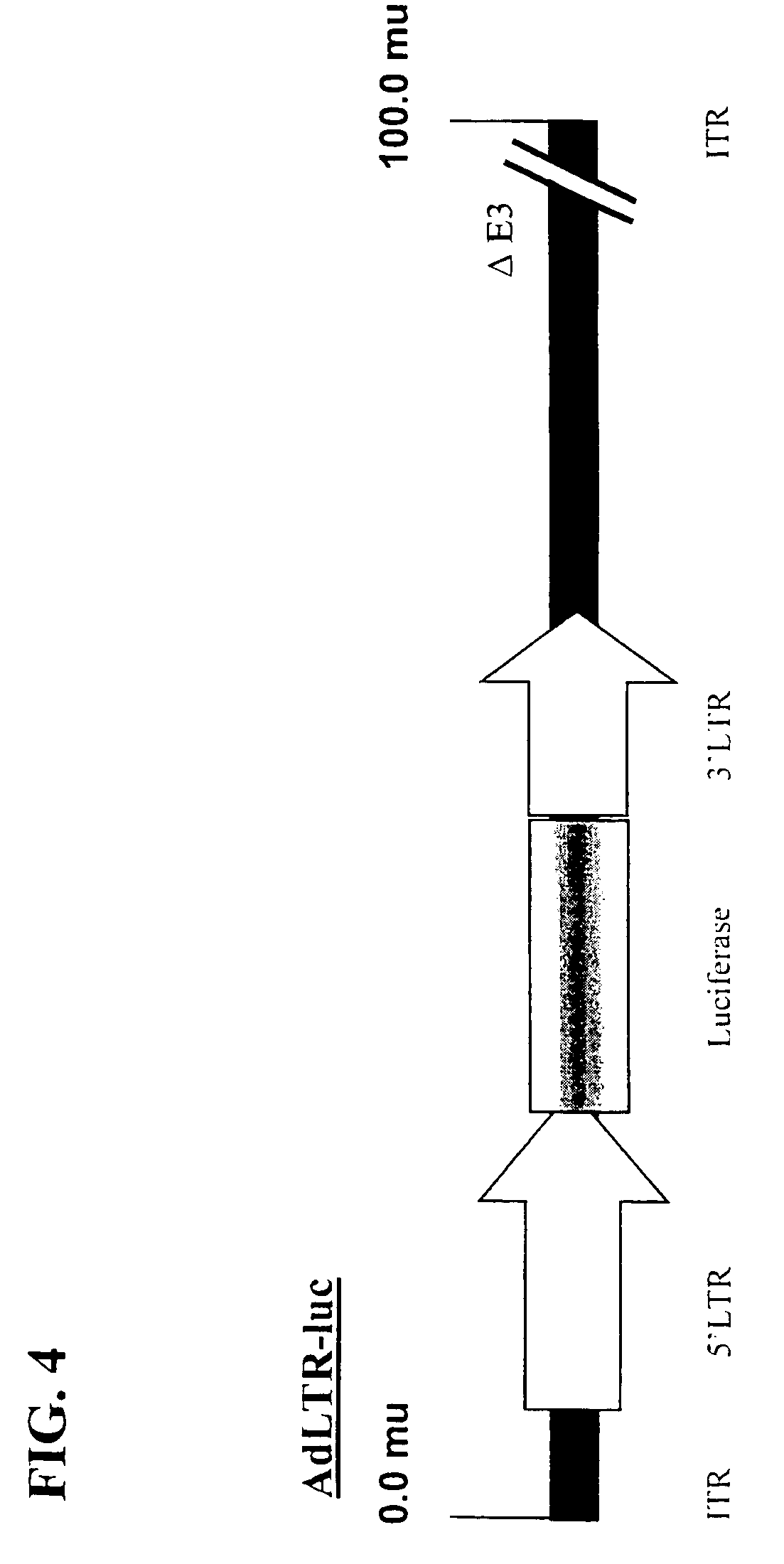
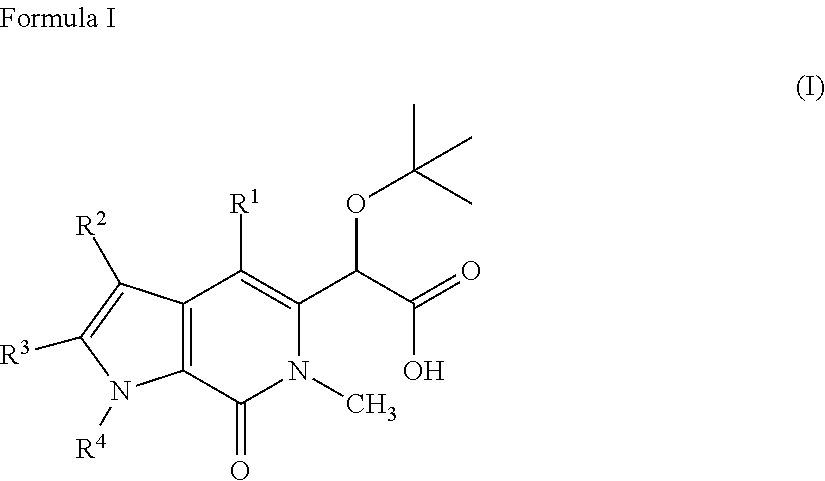
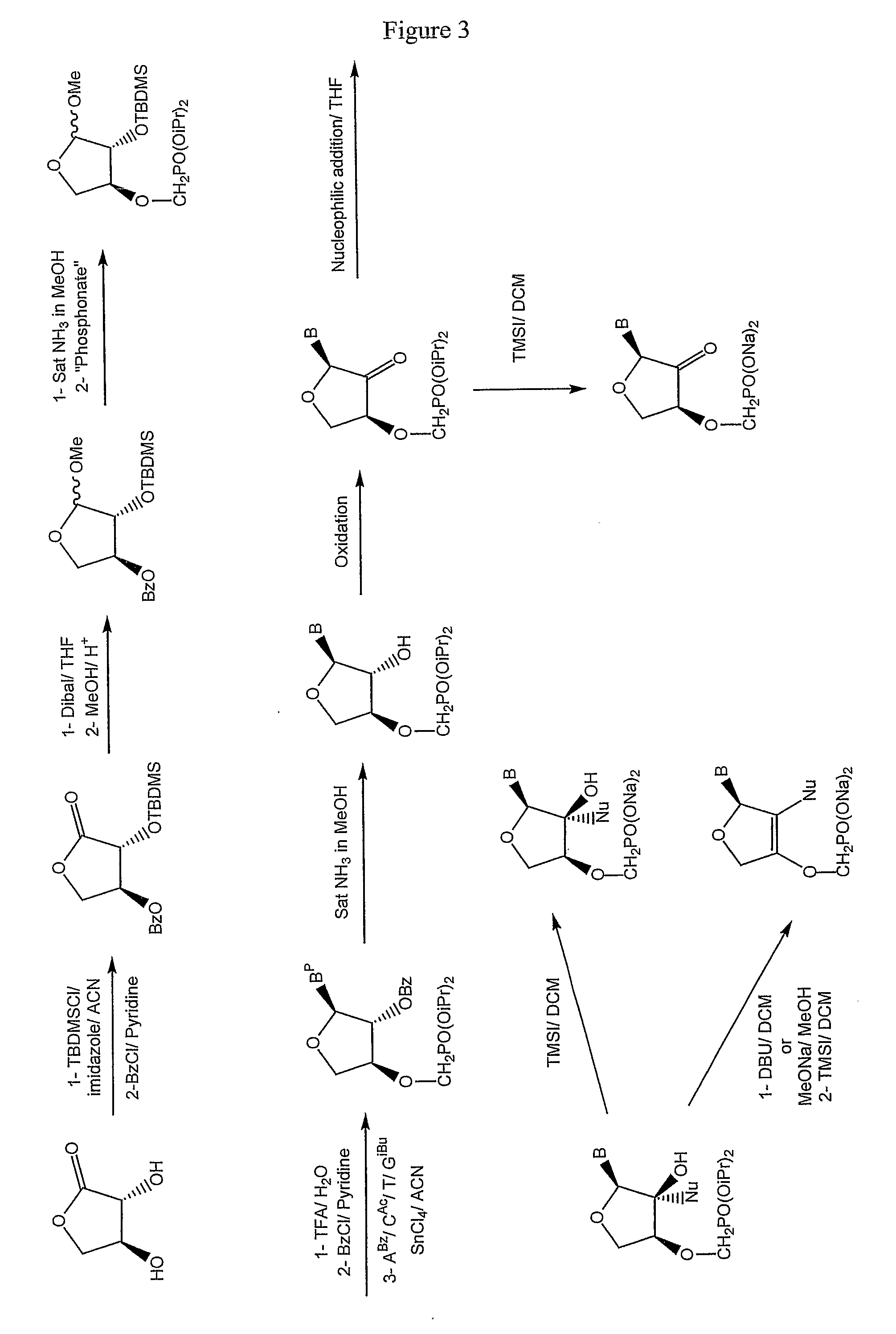
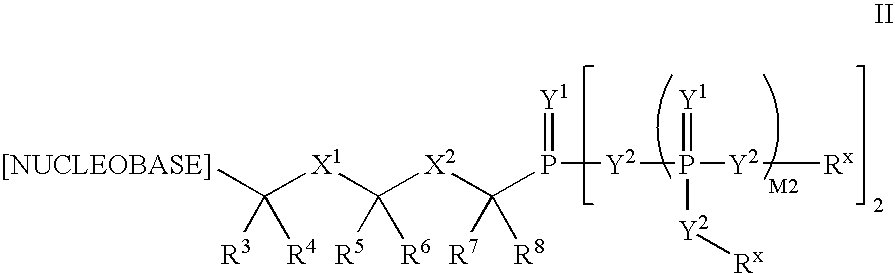Patents
Literature
42 results about "Viral Reverse Transcription" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The action of synthesizing a DNA product from a viral RNA template by a viral reverse transcriptase.
Hybrid adeno-retroviral vector for the transfection of cells
InactiveUS7052904B2BiocideGenetic material ingredientsViral Reverse TranscriptionNucleic acid sequencing
An adenovirus, including adenoviral capsid proteins, and a replication-defective adenoviral vector that includes a 5′ retroviral LTR nucleic acid sequence, a 3′ retroviral LTR nucleic acid sequence, a nucleic acid sequence encoding a portion of a retroviral envelope protein adjacent to either the 5′ LTR or the 3′ LTR nucleic acid sequence, a retroviral packaging sequence and a nucleic acid sequence encoding a transgene located between the 5′ LTR and the 3′ LTR is provided. Host cells infected with this adenovirus are also provided. An adenoviral vector is provided that includes an adenoviral polynucleotide sequence comprising a nucleic acid encoding a transgene, a retroviral packaging signal, a 5′ and a 3′ retroviral LTR, and a portion of a retroviral envelope polypeptide, wherein the adenoviral polynucleotide sequence does not encode one or more of E1, E3 or E4. A method for transforming a cell is also provided using a virus or a vector of the invention, as is a method for introducing a transgene into a cell that is not able to produce viral particles with a single viral vector. A method is also provided for preventing or treating disorder in a subject using the adenoviral vectors of the invention. A pharmaceutical composition is also provided that includes an adenoviral vector of the invention and a pharmaceutically acceptable carrier.
Owner:DEPT OF HEALTH & HUMAN SERVICES US SEC THE
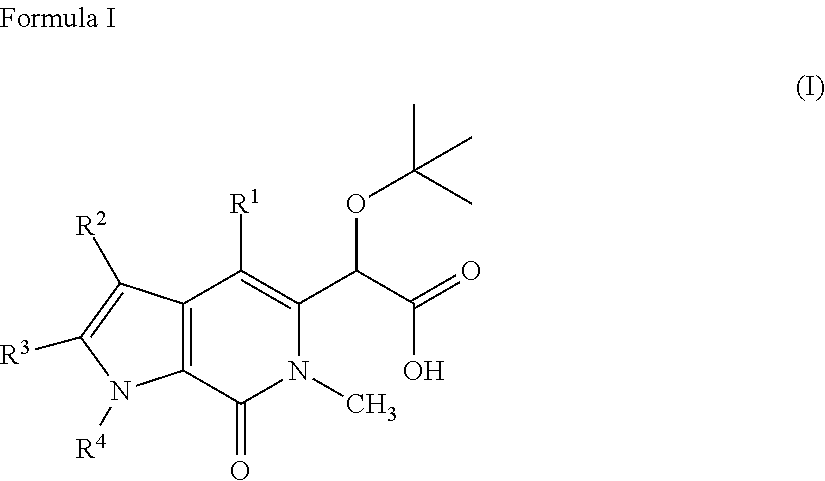
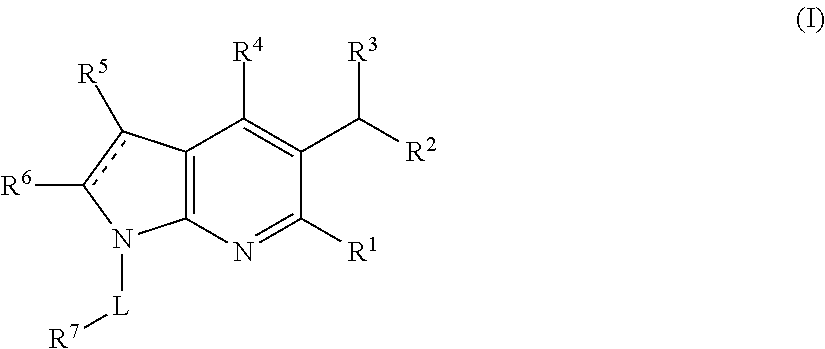
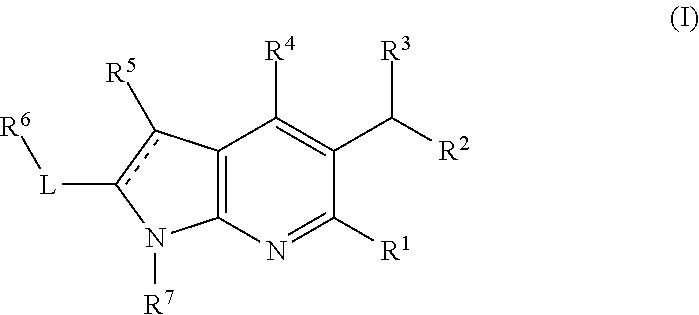
Pyrrolopyridinone compounds and methods for treating HIV
Provided are compounds and pharmaceutically acceptable salts thereof, their pharmaceutical compositions, their methods of preparation, and their use for treating viral infections mediated by a member of the retrovirus family of viruses such as the Human Immunodeficiency Virus (HIV).
Owner:VIIV HEALTHCARE UK LTD
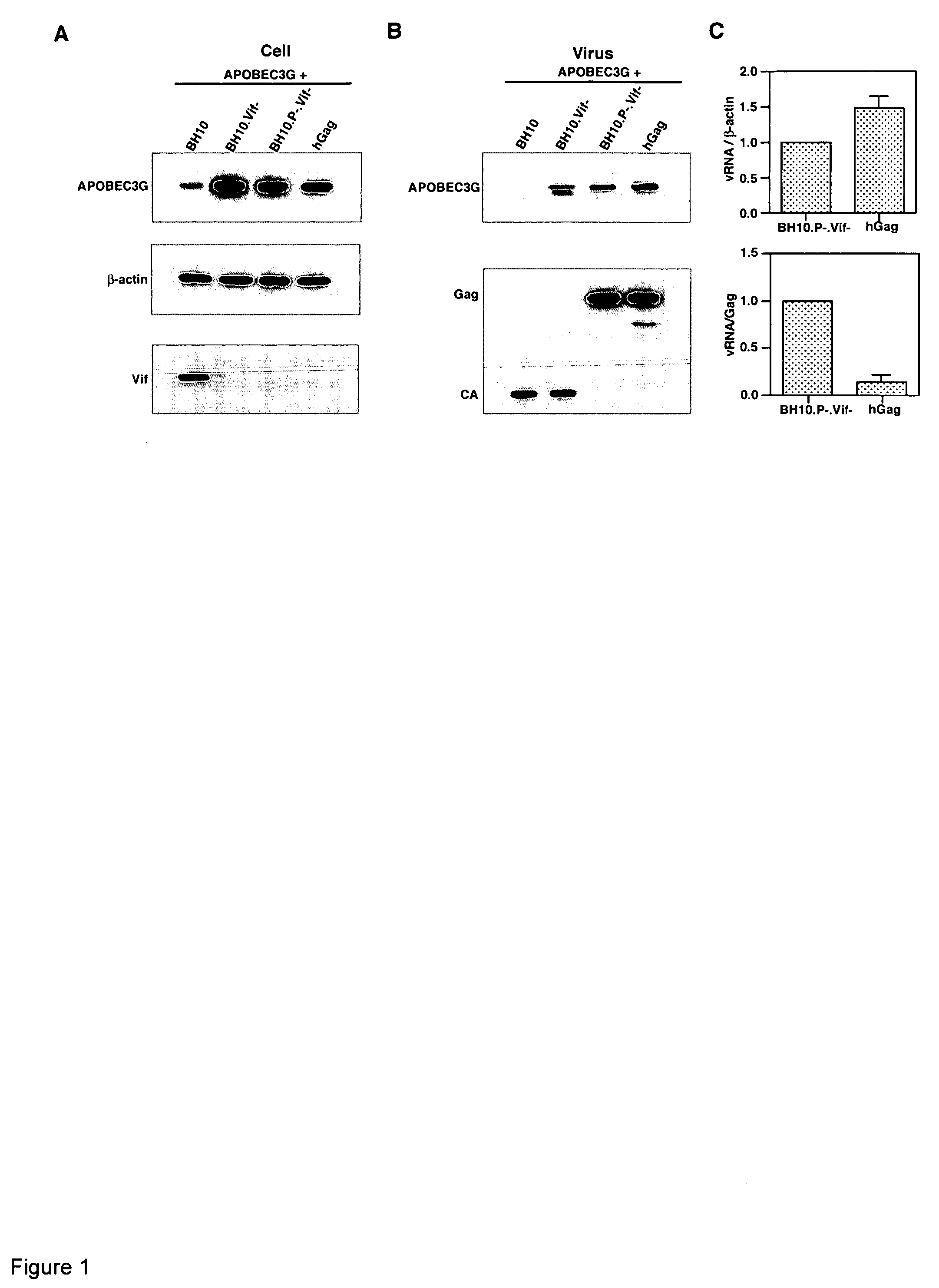
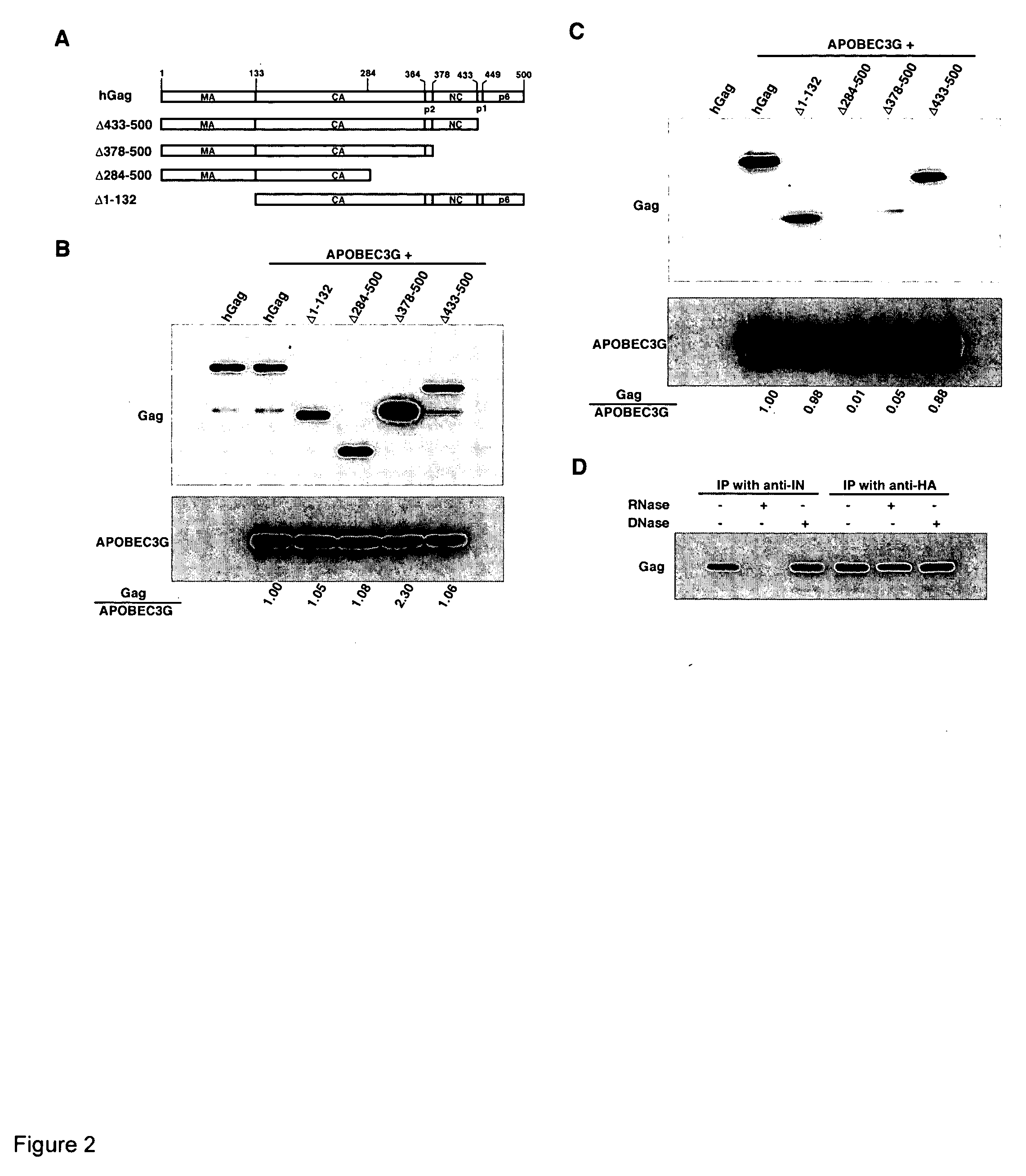
Inhibition of the tRNALys3-primed initiation of reverse transcription in HIV-1 by APOBEC3G
InactiveUS20060002951A1Inhibiting and reducing Vif-dependent inhibitionHigh affinityHydrolasesPeptide/protein ingredientsViral Reverse TranscriptionViral infection
The present invention generally relates to the field of antiviral therapy. More specifically, the present invention relates to the inhibition of the tRNALys3-primed initiation of reverse transcription in viruses by APOBEC3G. The present invention further relates to a method of treating or preventing viral infections by inhibiting tRNALys3 annealing and / or priming on a viral genome thereby reducing viral replication. More particularly, the present invention relates to the use of APOBEC3G, fragments or derivatives thereof for treatment or prophylaxis of HIV-1 infection and related lentivirus infections.
Owner:MCGILL UNIV
Compositions and Methods for RT-PCR
ActiveUS20140199699A1Rapid and efficient amplificationHigh detection sensitivityMicrobiological testing/measurementTrehaloseBiology
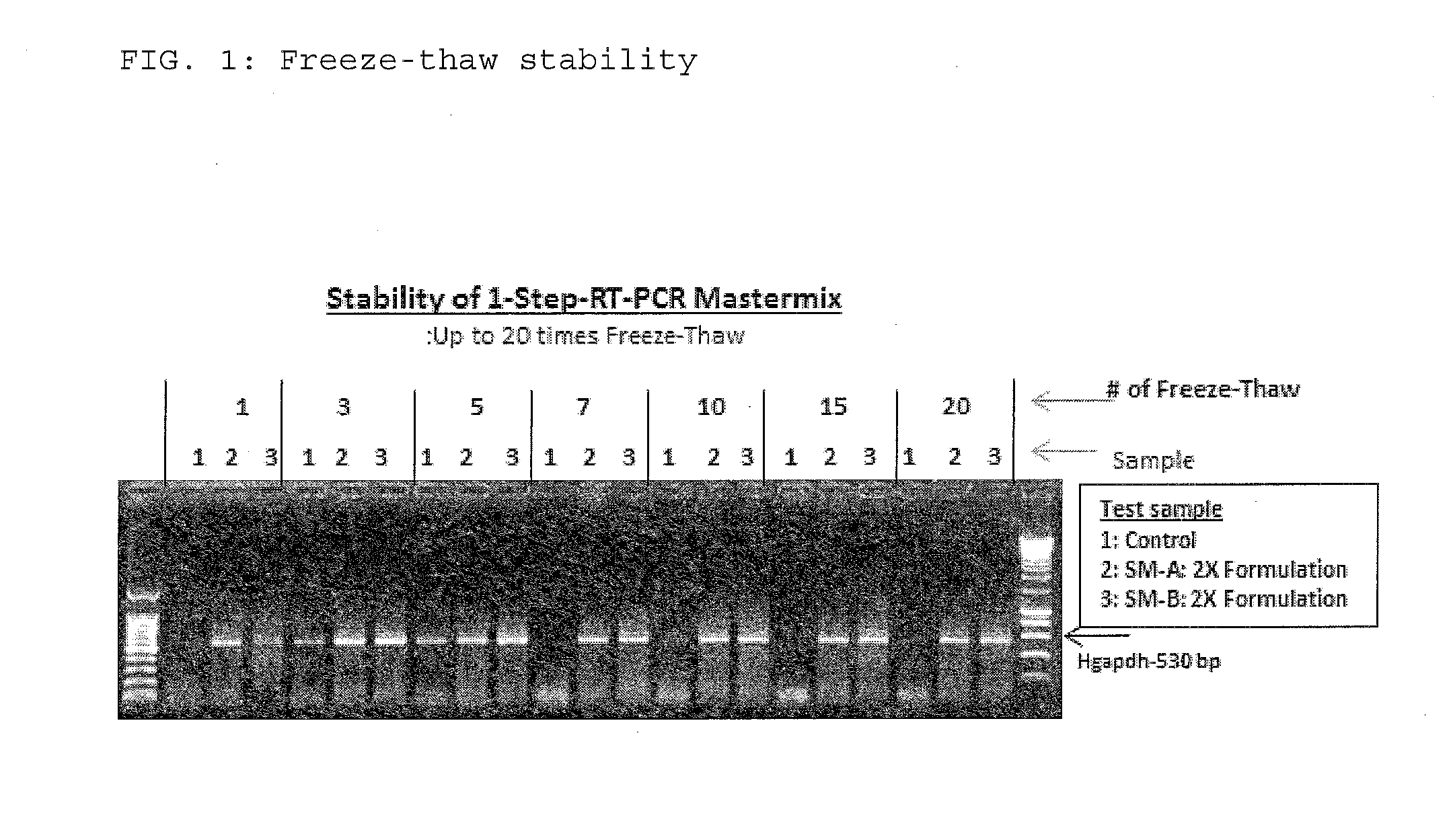
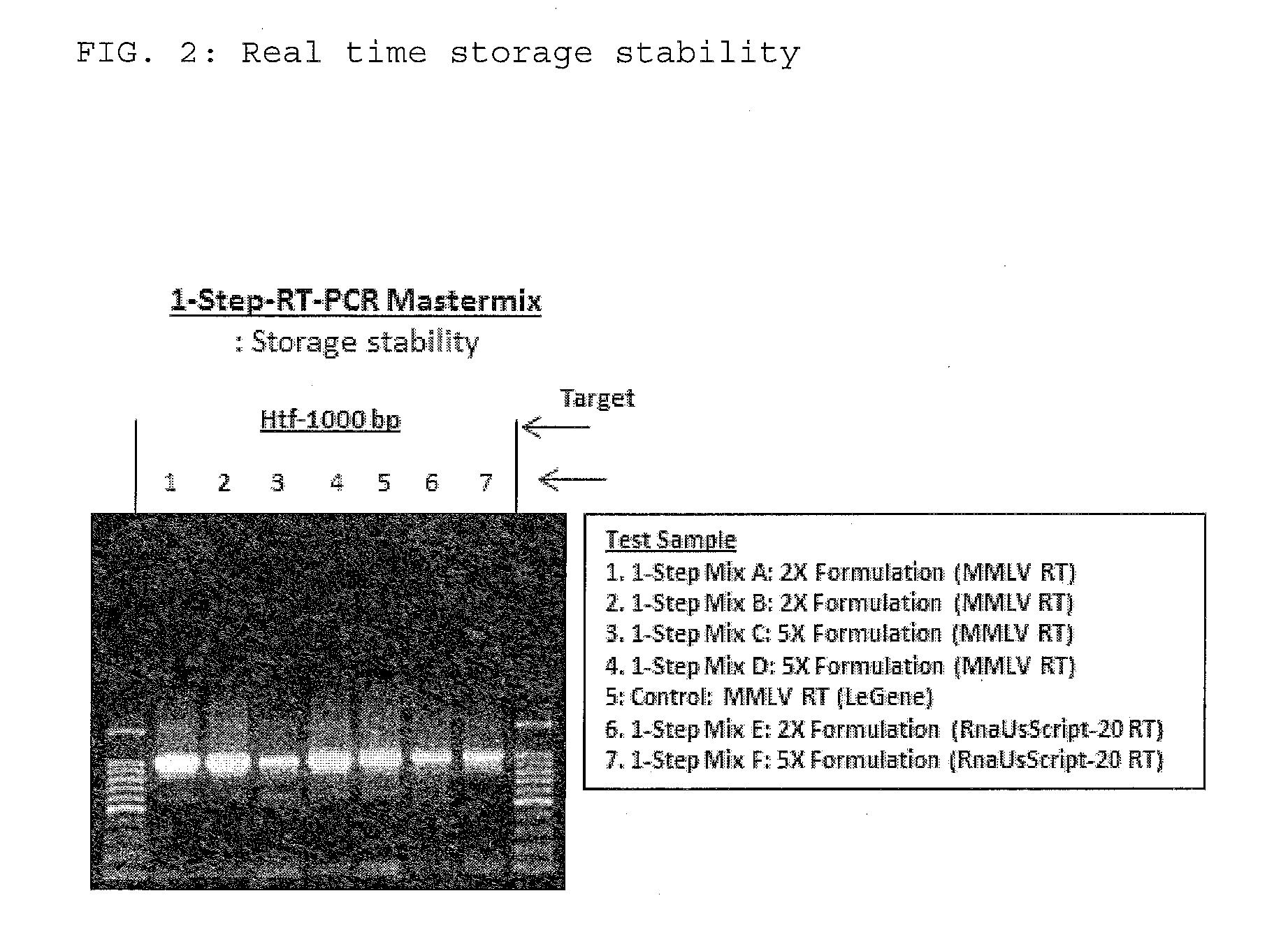
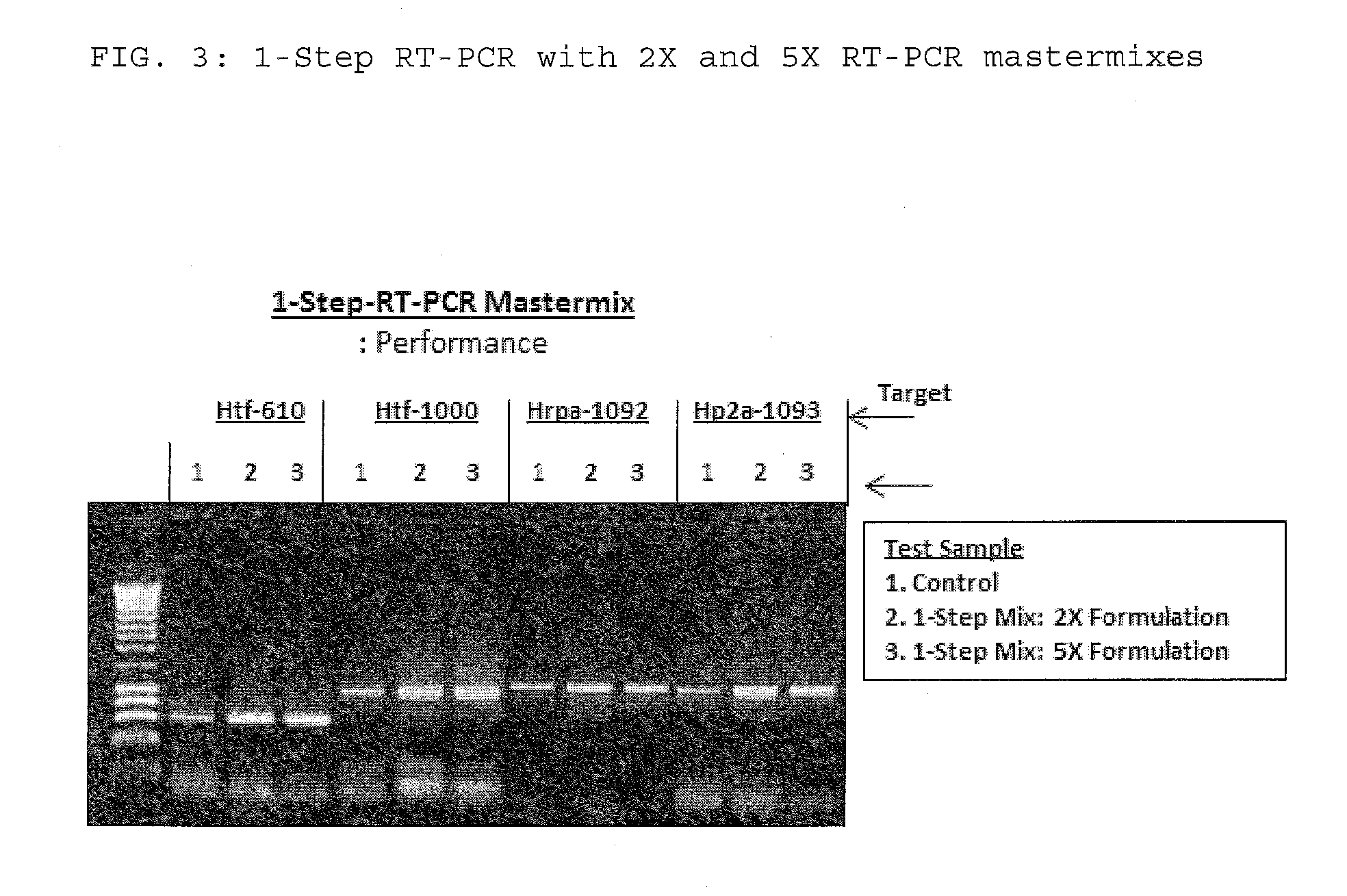
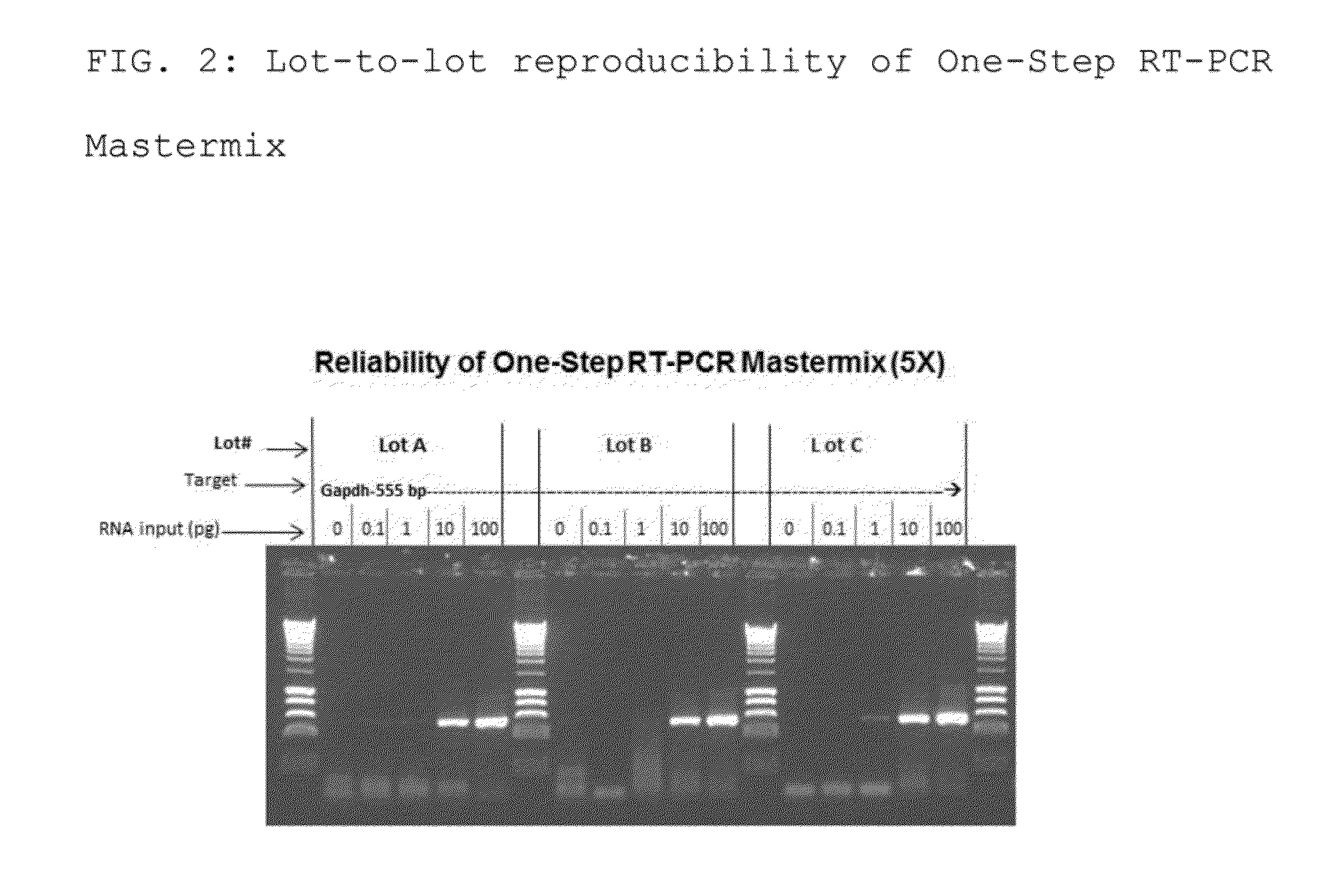
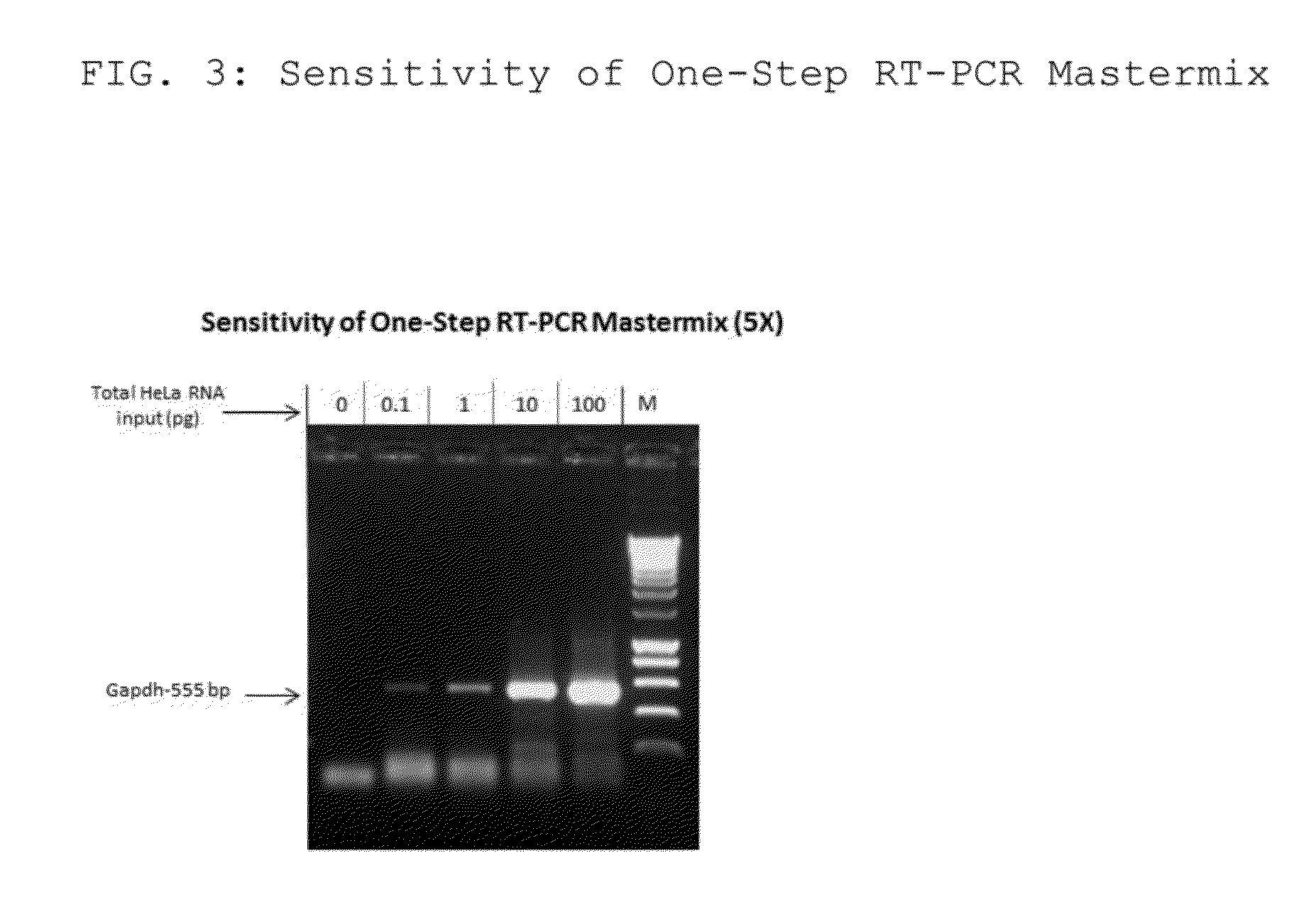
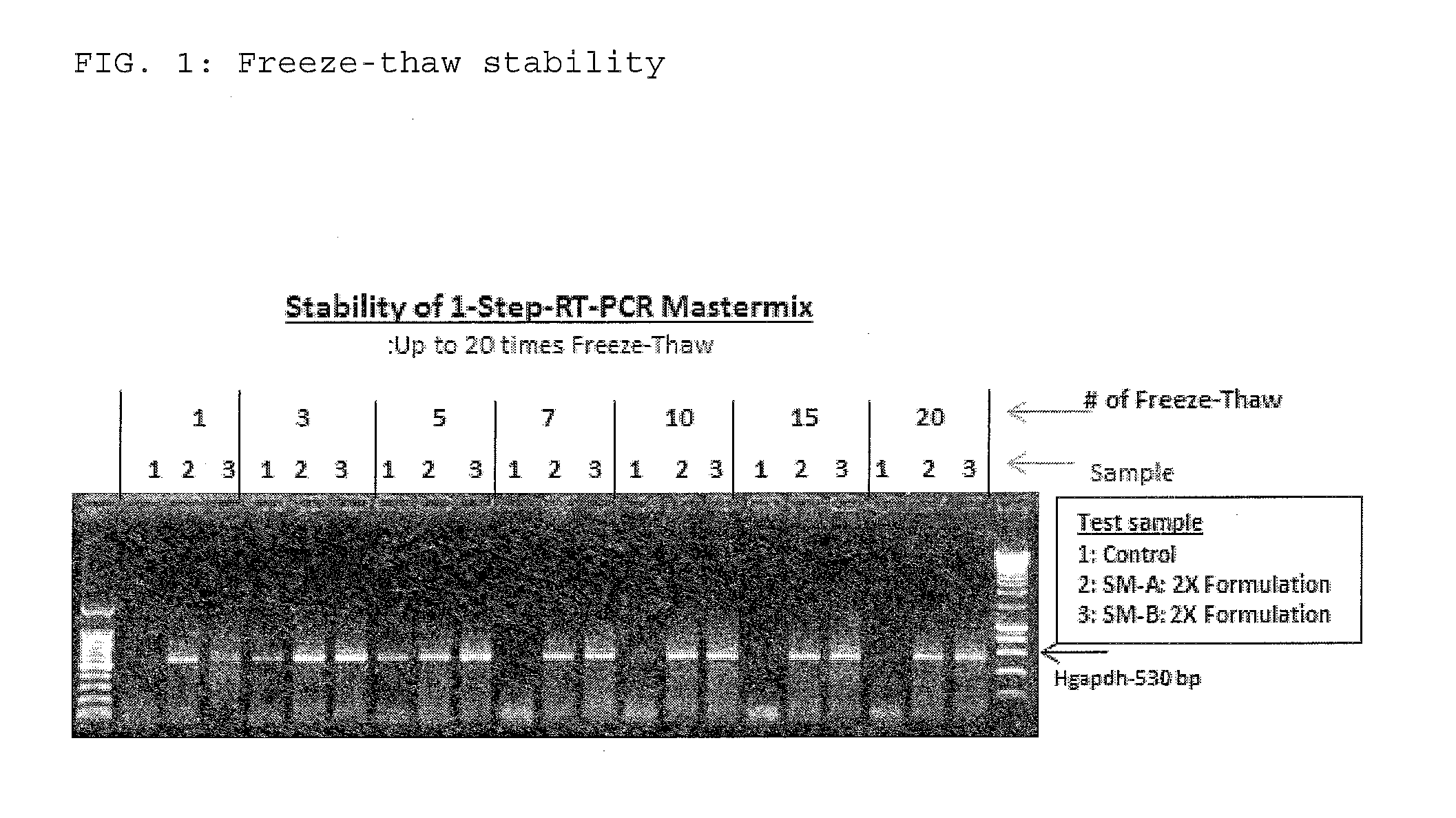
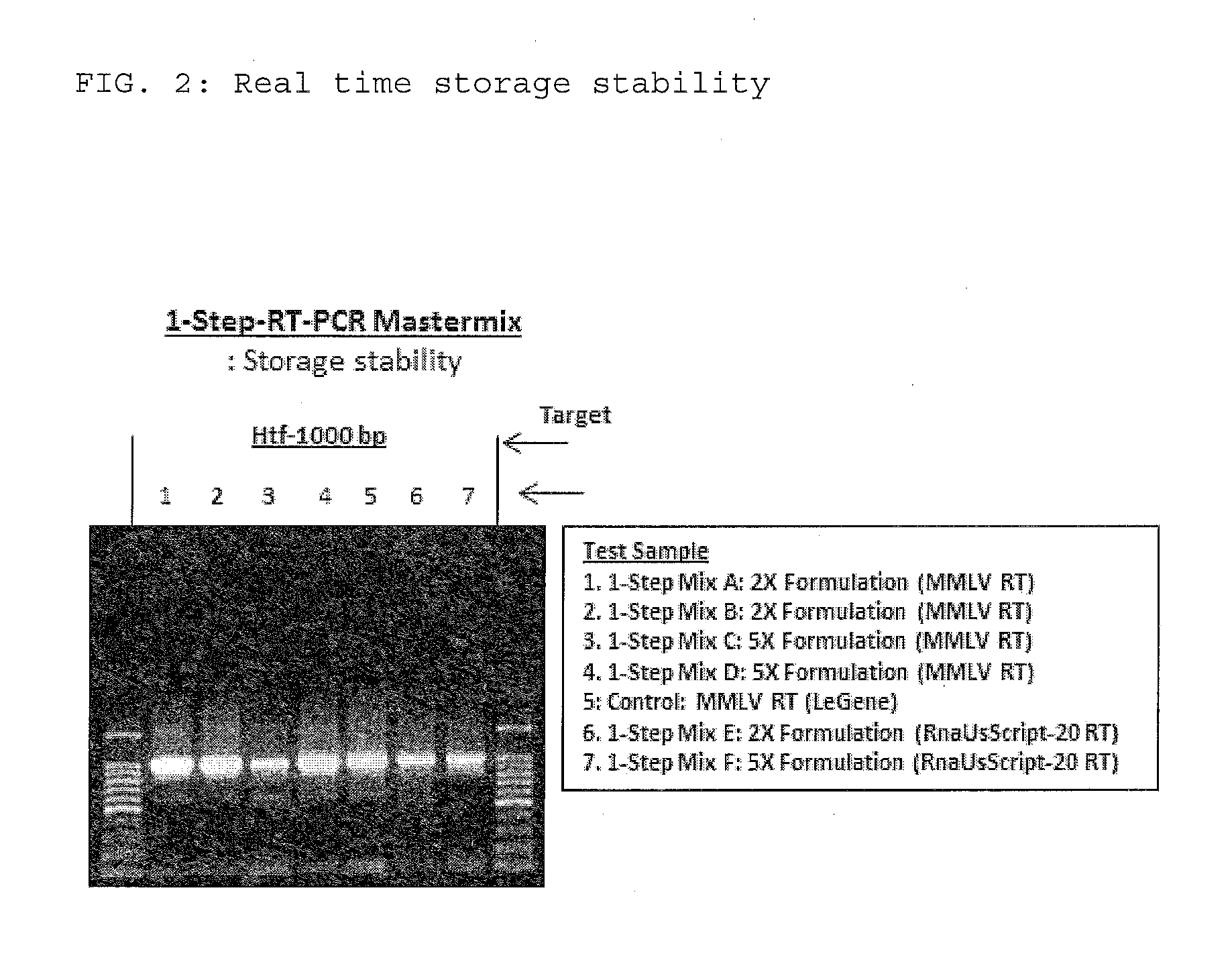
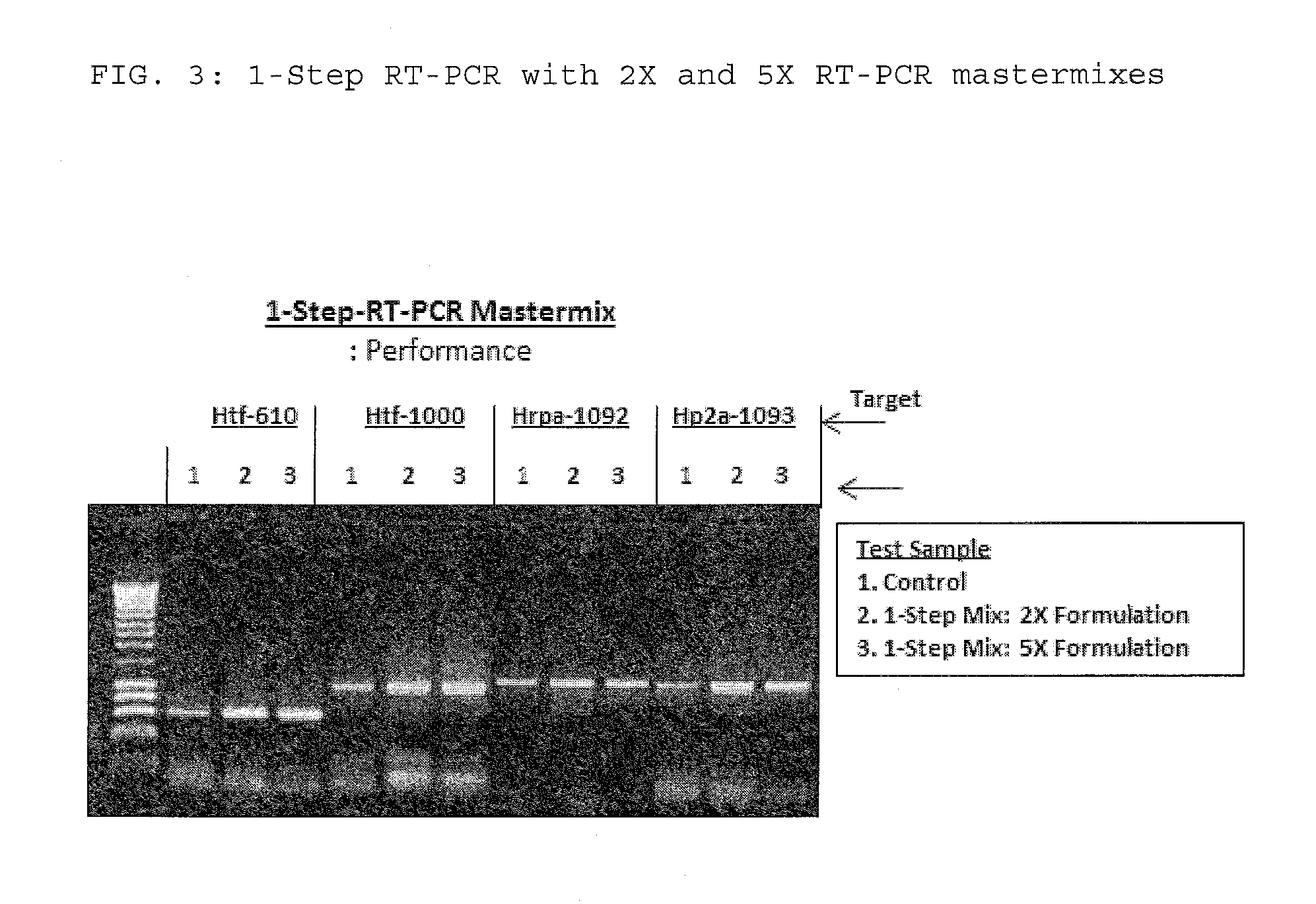
The present invention relates to methods and compositions having trehalose and DNA polymerase for facilitating the rapid and efficient amplification of nucleic acid molecules and the detection and quantitation of RNA molecules, and for increasing the detection sensitivity and reliability through generation of secure cDNA molecules prior to gene-specific primer dependent amplification. The reagent mixture comprises a ready to use reagent solution, wherein the solution comprises: (a) trehalose in a concentration between about 5% and about 35%; (b) a viral reverse transcriptase; and (c) at least one DNA polymerases, in a buffer suitable for use in a reverse transcription reaction, wherein the buffer comprises a co-factor metal ion and nucleoside triphosphates.
Owner:LEE JUN EUIHUM
Use of endoperoxides for the treatment of infections caused by flaviviridae, including hepatitis C, bovine viral diarrhea and classical swine fever virus
InactiveUS20050059647A1Useful in treatmentBiocidePeptide/protein ingredientsAbnormal tissue growthBovine Viral Diarrhea Viruses
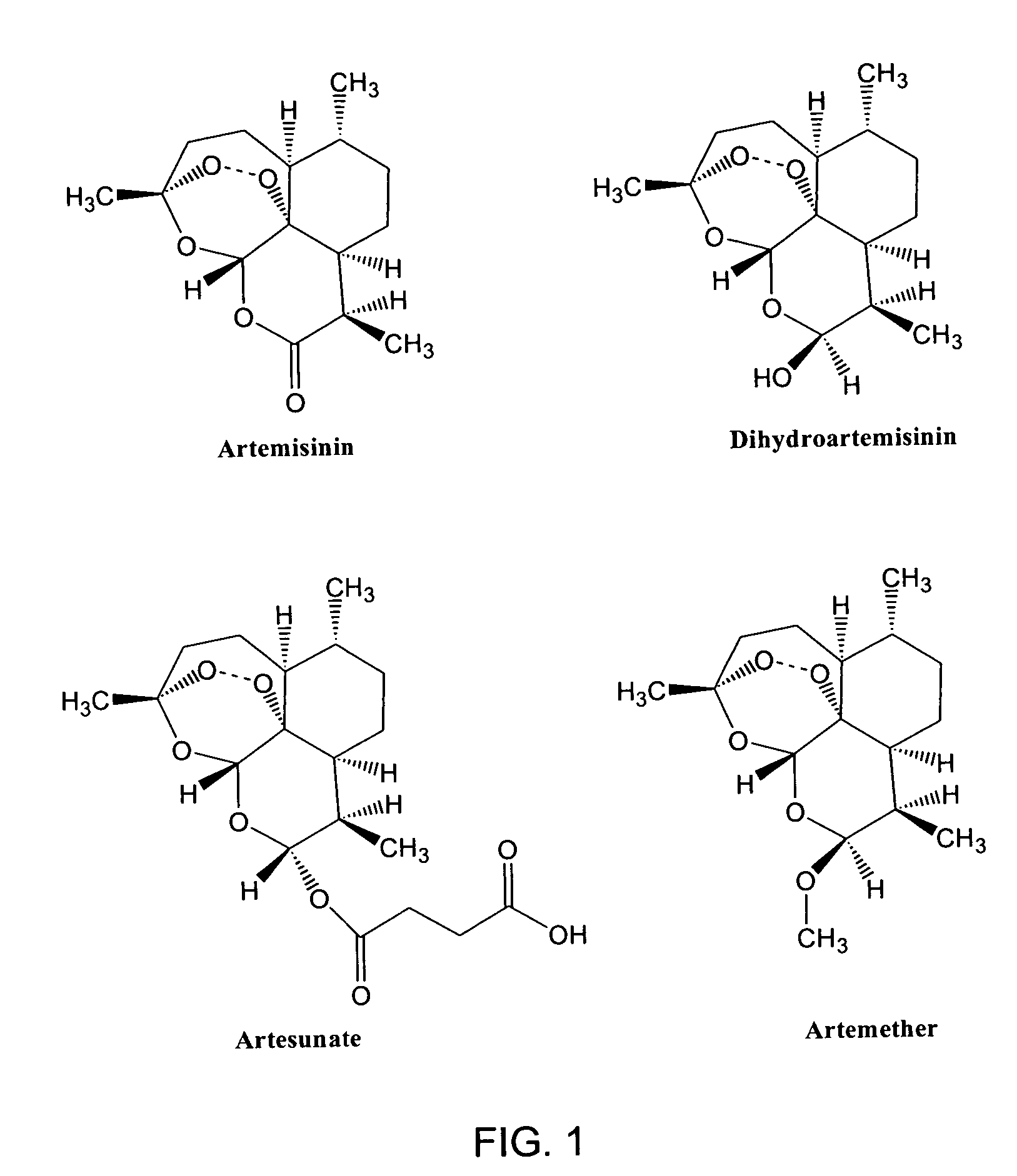
The use of sesquiterpenes and, in particular sesquiterpene lactone endoperoxides, such as artemisinin and analogs thereof, for the treatment of hepatitis C virus infections. Artemisinin, analogs of artemsisnin and some crude Artemisia extracts were tested in vitro against DNA-viruses, retro-viruses and Flavivirida, (an important family of human and animal RNA pathogens). These compounds were also screened for anti-tumor activity. Strong activity of artemisinin was noticed against the bovine viral diarrhea virus (BVDV). As pestiviruses, such as BVDV, share many similarities with hepatitis C virus (HCV), we can conclude that endoperoxides in general and artemisinin more specificly have efficacy as treatments for hepatitis C viral infections.
Owner:KEMIN FOODS L C
Nucleobase phosphonate analogs for antiviral treatment
ActiveUS20050059637A1Increasing cellular accumulationImprove retentionBiocideOrganic active ingredientsHigh concentrationAcquired immunodeficiency
The present invention provides novel compounds with activity against infectious viruses. The compounds of the invention may inhibit retroviral reverse transcriptases and thus inhibit the replication of the virus. They are useful for treating human patients infected with a human retrovirus, such as human immunodeficiency virus (strains of HIV-1 or HIV-2) or human T-cell leukemia viruses (HTLV-I or HTLV-II) which results in acquired immunodeficiency syndrome (AIDS) and / or related diseases. The present invention also relates generally to the accumulation or retention of therapeutic compounds inside cells. The invention is more particularly related to attaining high concentrations of active metabolite molecules in HIV infected cells. Intracellular targeting may be achieved by methods and compositions which allow accumulation or retention of biologically active agents inside cells. Such effective targeting may be applicable to a variety of therapeutic formulations and procedures.
Owner:GILEAD SCI INC
Hybrid adeno-retroviral vector for the transfection of cells
InactiveUS20090258935A1VectorsGenetic material ingredientsViral Reverse TranscriptionNucleic acid sequencing
An adenovirus, including adenoviral capsid proteins, and a replication-defective adenoviral vector that includes a 5′ retroviral LTR nucleic acid sequence, a 3′ retroviral LTR nucleic acid sequence, a nucleic acid sequence encoding a portion of a retroviral envelope protein adjacent to either the 5′ LTR or the 3′ LTR nucleic acid sequence, a retroviral packaging sequence and a nucleic acid sequence encoding a transgene located between the 5′ LTR and the 3′ LTR is provided. Host cells infected with this adenovirus are also provided. An adenoviral vector is provided that includes an adenoviral polynucleotide sequence comprising a nucleic acid encoding a transgene, a retroviral packaging signal, a 5′ and a 3′ retroviral LTR, and a portion of a retroviral envelope polypeptide, wherein the adenoviral polynucleotide sequence does not encode one or more of E1, E3 or E4. A method for transforming a cell is also provided using a virus or a vector of the invention, as is a method for introducing a transgene into a cell that is not able to produce viral particles with a single viral vector. A method is also provided for preventing or treating disorder in a subject using the adenoviral vectors of the invention. A pharmaceutical composition is also provided that includes an adenoviral vector of the invention and a pharmaceutically acceptable carrier.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY DEPARTMENT OF HEALTH AND HUMAN SERVICES
Reverse transcription loop-mediated isothermal amplification test kit of hog cholera virus and application thereof
InactiveCN104651535ASimple and fast operationEasy accessMicrobiological testing/measurementMicroorganism based processesViral Reverse TranscriptionSpecific detection
The invention discloses a reverse transcription loop-mediated isothermal amplification test kit of a hog cholera virus and an application thereof. The test kit comprises an RT-LAMP primer, a 2*reaction buffer solution, an EM, a fluorescence visual detection reagent, ultrapure water and a hog cholera virus RNA template. The RT-LAMP primer comprises outer primers F3 and B3, inner primers FIP and BIP and a loop primer LF. The specific detection, the sensibility detection and the fluorescence visual detection prove that by adopting the RT-LAMP detecting method, the hog cholera virus can be specifically detected, the reaction can be real-time monitored, the copy number of the hog cholera virus can be quantitatively determined, the detecting result can be rapidly and accurately obtained, so that the simple, rapid and reliable detection of the hog cholera virus are facilitated.
Owner:GUANGXI VETERINARY RES INST
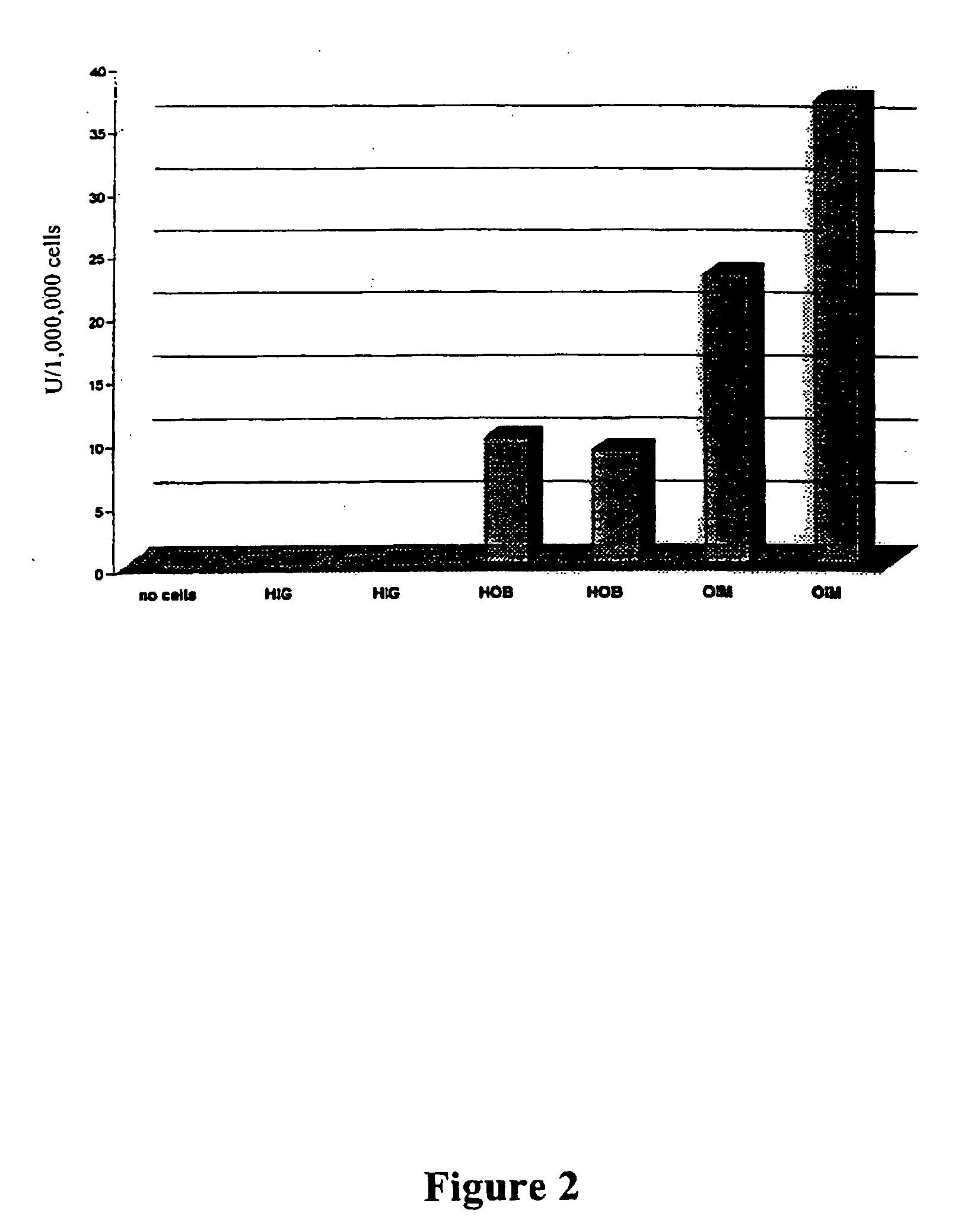
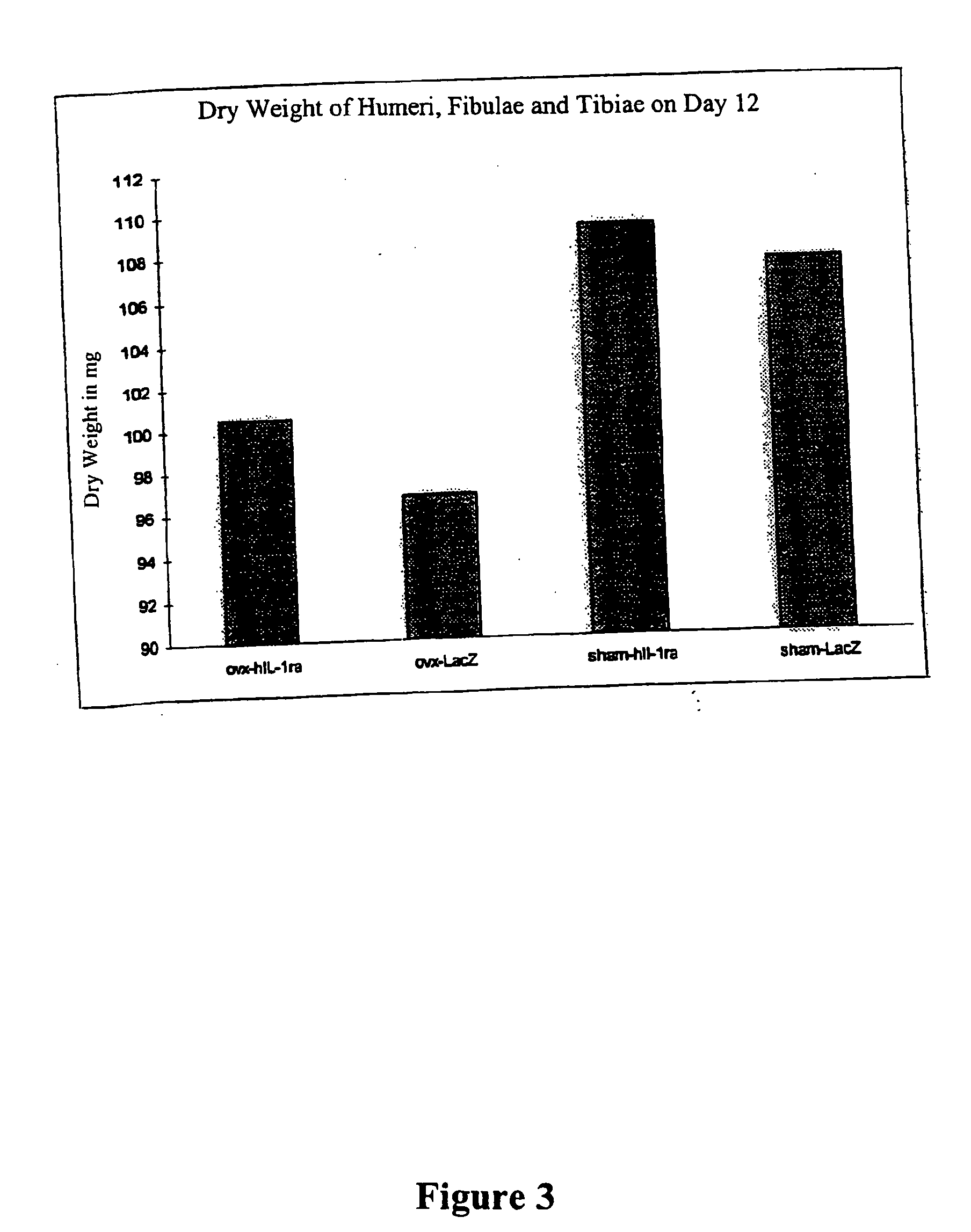
Viral and non-viral vectors as vehicles for delivering transgenes for treating bone pathologies
The present invention relates to a method for treating bone pathologies comprising delivering a viral or non-viral delivery vehicle comprising genetic information (e.g. a transgene) encoding a therapeutic osteoinductive factor to target cells in vivo enabling the cells to produce the osteoinductive factor at the site of the bone pathology. The delivery is achieved by a simplified method which does not require cumbersome ex vivo techniques or additional matrix or scaffolding agents. Such viral and non-viral delivery vehicles of the present invention are derived from the following nonlimiting examples: adenoviruses, adeno-associated viruses, retroviruses, herpes simplex viruses, liposomes, and plasmids. The osteoinductive factors include, but are not limited to, growth factors, cytokines, growth factor inhibitors and cytokine inhibitors.
Owner:BALTZER AXEL W +2
Detection kit and detection method for siniperca chuatsi rhabdoviruses
ActiveCN104032038AQuick checkExtensive use of disease surveillanceMicrobiological testing/measurementPositive controlAquatic animal
The invention belongs to the technical field of detection and diagnosis of aquatic animal pathogeny, and discloses a detection kit and detection method for siniperca chuatsi rhabdoviruses. The reverse transcription-polymerase chain reaction type rapid detection kit for the siniperca chuatsi rhabdoviruses is filled with a 5* reverse transcription buffer solution, reverse transcriptase, a random primer, 1.0 mL of 2*reaction mixed buffer solution, a preferably-designed sense primer, a preferably-designed reverse primer, Taq DNA polymerase, a positive control solution, a negative control solution and ddH2O. The novel reverse transcription-polymerase chain reaction type rapid detection kit and detection method for the siniperca chuatsi rhabdoviruses overcome shortcomings of an existing detection method for the siniperca chuatsi rhabdoviruses, are practicable when applied to clinical diagnosis of the siniperca chuatsi rhabdoviruses, have the advantages of rapidity, accuracy and specificity, meet requirements of the clinical diagnosis, and provide convenience for detection of the siniperca chuatsi rhabdoviruses.
Owner:GUANGZHOU JINSHUI ANIMAL HEALTH PROD
Nucleobase phosphonate analogs for antiviral treatment
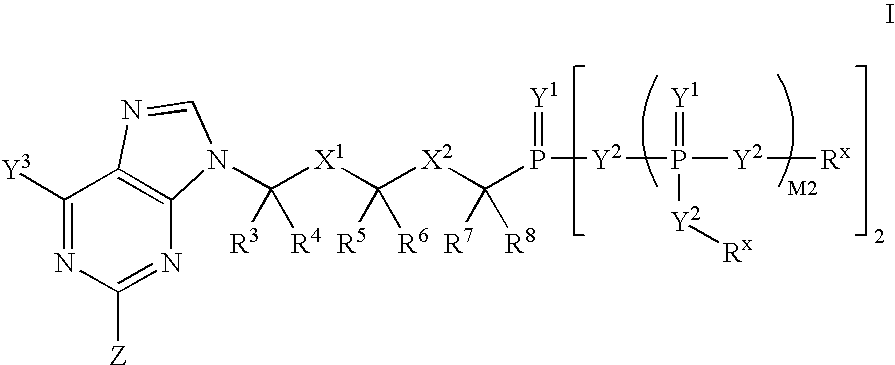
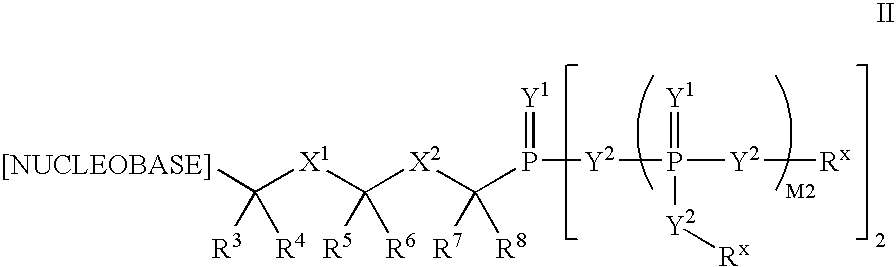
ActiveUS7579332B2Increasing cellular accumulation and retentionIncrease valueBiocideOrganic active ingredientsViral Reverse TranscriptionHuman T cell leukemia virus
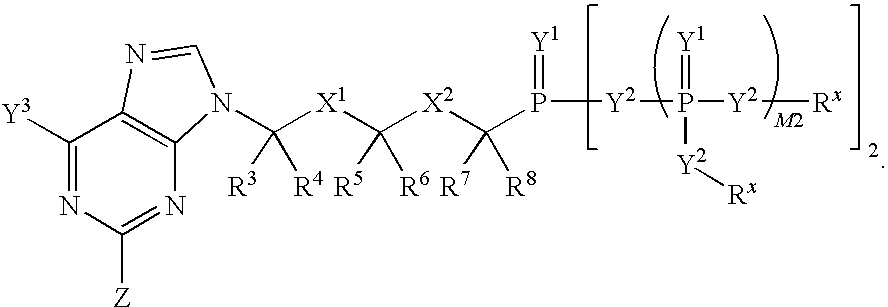
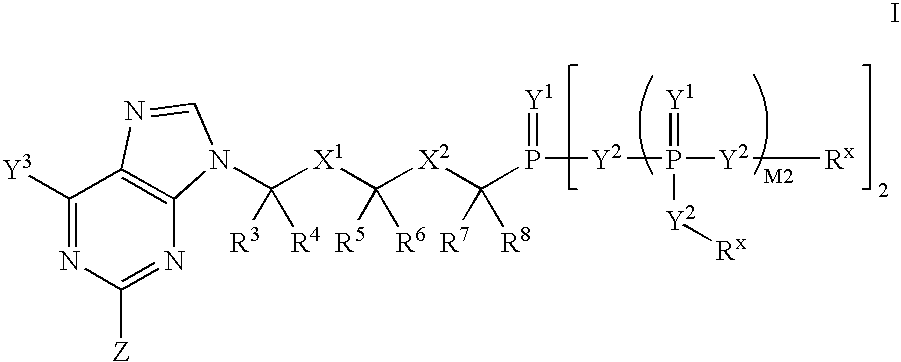
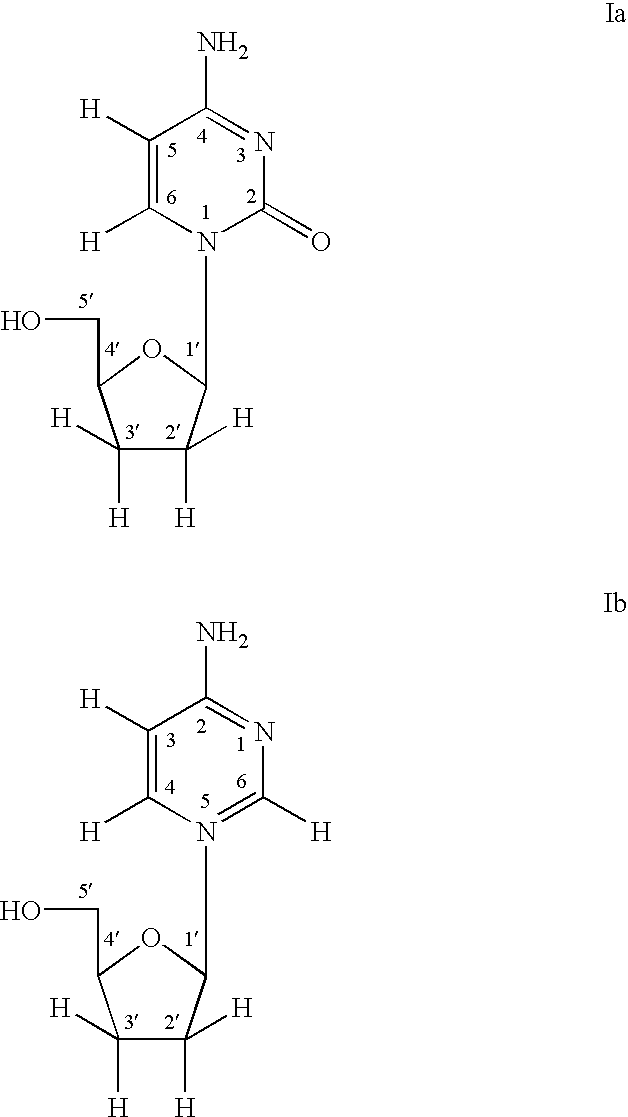
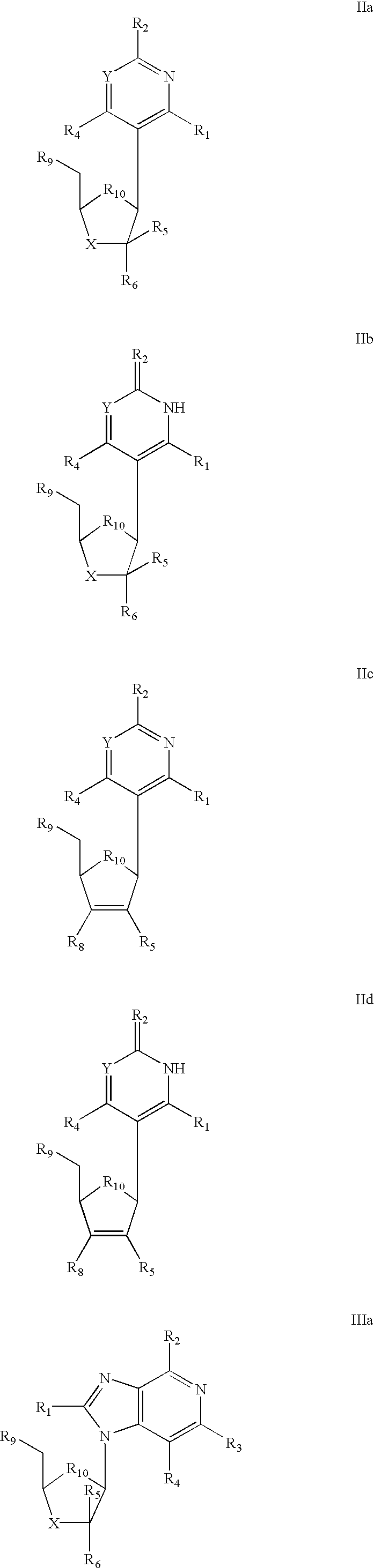
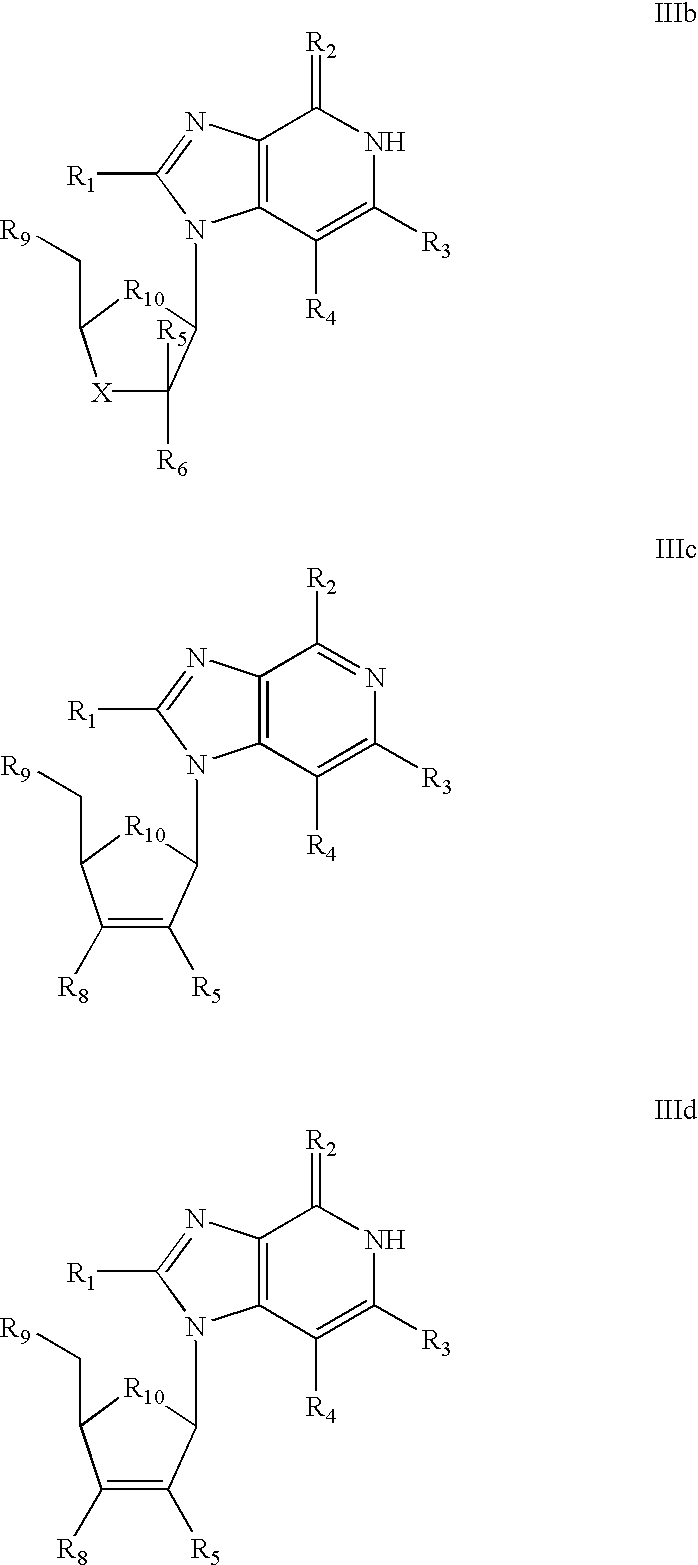
The invention provides compounds with activity against infectious diseases. The compounds of the invention may inhibit retroviral reverse transcriptases and thus inhibit the replication, of the virus. The compounds of the invention may be useful for treating human patients infected with a human retrovirus, such as human immunodeficiency virus (strains of HIV-l or HIV-2) or human T-cell leukemia virus (HTLV-1 or HTLV-II) which results in acquired immunodeficiency syndrome (AIDS) and / or related diseases. Representative of the invention is a compound of the following formula, with the substituents defined herein:
Owner:GILEAD SCI INC
Selective anti-viral nucleoside chain terminators
InactiveUS6914052B2Low toxicityPotent anti-viralSaccharide with heterocyclic radicalsBiocideImmunodeficiency virusPolymerase L
The present invention relates to dideoxynucleoside analog compounds containing a dideoxy ribofuranosyl moiety that exhibit selective anti-viral activity coupled with substantially low toxicity toward the host cells. In particular, the compounds according to the present invention show potent inhibition of the replication of the human immunodeficiency virus (HIV), while remaining substantially inert toward host cell DNA. Compounds according to the present invention exhibit primary utility as agents for inhibiting the growth or replication of retroviruses, particularly HIV. The compounds of the invention comprise a (2,3′-dideoxy-β-ribofuranosyl) ring coupled to a heterocyclic nucleobase that lacks an “O2 carbonyl”, that enables them to selectively react with and inhibit viral reverse transcriptase, while remaining substantially unreactive toward human DNA polymerases.
Owner:BOSTON COLLEGE
Drug combination for the treatment of viral diseases
InactiveUS6576622B1BiocideCarbohydrate active ingredientsThymidine analogueViral Reverse Transcription
This invention pertains to a method for treating a human with human immunodeficiency virus infection which comprises administering to the human a therapeutically effective amount of a thymidine analog, which analog acts as an inhibitor of viral reverse transcriptase necessary for viral replication of human immunodeficiency virus, and a thymidylate synthase inhibitor, or pharmaceutically acceptable salts thereof.
Owner:NEW JERSEY UNIVESITY OF MEDICINE & DENTISTRY OF
Pyrrolopyridinone Compounds And Methods For Treating HIV
Provided are compounds and pharmaceutically acceptable salts thereof, their pharmaceutical compositions, their methods of preparation, and their use for treating viral infections mediated by a member of the retrovirus family of viruses such as the Human Immunodeficiency Virus (HIV).
Owner:VIIV HEALTHCARE UK LTD
Tilapia Lake Virus (TiLV) specificity RT-PCR detection kit and detection method
InactiveCN107760802AQuick checkExtensive use of epidemiological surveysMicrobiological testing/measurementMicroorganism based processesPositive controlViral Reverse Transcription
The invention belongs to the technical field of aquaculture and in particular relates to a tilapia lake virus (TiLV) specificity RT-PCR detection kit and a detection method. The TiLV reverse transcription-polymerase chain reaction rapid detection kit comprises 5*reverse transcription buffer solution, reverse transcriptase, a random primer, 2*reaction mixed buffer solution, preferred designed upstream and downstream primers, Taq DNA polymerase, positive control solution, negative control solution and ddH2O. The invention overcomes the defects of an existing TiLV detection method and provides anovel reverse transcription-polymerase chain reaction rapid detection kit and a detection method; and the detection kit and the detection method are not only practicable in TiLV clinical diagnosis butalso have the advantages of speediness, accuracy, specificity and sensitivity, clinical diagnosis requirements are met, and convenient conditions are provided for TiLV detection.
Owner:广州利洋水产科技股份有限公司
Method for fast inspecting HBV, HCV, HIV, TP and RV simultaneouslly
InactiveCN1940089ASensitive detectionMicrobiological testing/measurementPathogenic microorganismBiological body
A method for inspecting HBV, HCV, HIV, TP and RV simultaneously and rapidly is carried out by extracting virus inspection template, RNA virus reversed transcripting, PCR amplifying for five peccant microbe genes in the one same reactive system, inspecting by agarose gel electrophoresis method, positioning several peccant microbe gene sensitive target genes, designing multiple PCR nested or semi-nested primer and inspecting sensitively and rapidly. It can be used to inspect clinical human body or animal blood or tissue sample.
Owner:鲍玉洲 +1
Compositions and Methods for RT-PCR
InactiveUS20160097086A1Rapid and non-bias productionAccelerated programMicrobiological testing/measurementBiologyNucleoside triphosphate
The present invention relates to compositions and methods having propylene glycol and DNA polymerase for facilitating the rapid and efficient amplification of nucleic acid molecules and the detection and quantitation of RNA molecules, and for increasing the detection sensitivity and reliability through generation of secure cDNA molecules prior to gene-specific primer dependent amplification. The reagent mixture comprises a ready to use reagent solution, wherein the solution comprises: (a) propylene glycol in a concentration between about 20% and about 50%; (b) a viral reverse transcriptase; and (c) at least one DNA polymerases, in a buffer suitable for use in a reverse transcription reaction, wherein the buffer comprises a co-factor metal ion and nucleoside triphosphates.
Owner:LEE JUN EUIHUM
Reverse transcription loop-mediated isothermal amplification (RT-LAMP) kit for porcine kobuvirus and application thereof
InactiveCN105821160AEasy accessSimple and fast operationMicrobiological testing/measurementMicroorganism based processesSpecific detectionViral Reverse Transcription
The invention discloses a visual reverse transcription loop-mediated isothermal amplification (RT-LAMP) kit for the porcine kobuvirus and application thereof. The kit comprises an RT-LAMP primer, 2*reaction buffer, effective microorganisms (EM), a fluorescent visual detection reagent, ultrapure water and a porcine kobuvirus ribonucleic acid (RNA) template, wherein the RT-LAMP primer comprises outer primers F3 and B3, inner primers FIP and BIP and loop primers LF and LB. The kit is applied to detecting the pathological tissues of the porcine kobuvirus and virus-containing excrement. Specific detection and sensitivity detection verify that the RT-LAMP kit provided by the invention can monitor reaction in real time and quantitatively detect the copy number of the porcine kobuvirus, quickly and accurately obtain the detection results and bring convenience for simply, conveniently, quickly and reliably detecting the porcine kobuvirus.
Owner:广西农垦永新畜牧集团金光有限公司 +1
Hepatitis B virus multi-locus genotypic resistance mutation detection method
InactiveCN101698891AEliminate the effects ofHigh sensitivityMicrobiological testing/measurementMicroorganism based processesViral Reverse TranscriptionMutation detection
The invention discloses a hepatitis B virus multi-locus genotypic resistance mutation detection method. A single tube nest type polymerase gene amplification method for detecting reverse transcriptase full gene sequence of virus includes that nest type PCR amplification is carried out in a single tube for twice, products of a first group PCR reaction are all used as templates of a second group PCR reaction by adjusting concentration of internal and external primers, and finally nest type PCR reaction is completed in the same single tube. The invention ensures specificity and economy of detection while obviously improving detection sensibility and has the detection range over a hundred million difference viral load.
Owner:THE FIFTH MEDICAL CENT OF CHINESE PLA GENERAL HOSPITAL
Azaindole compounds and methods for treating HIV
Owner:VIIV HEALTHCARE UK LTD
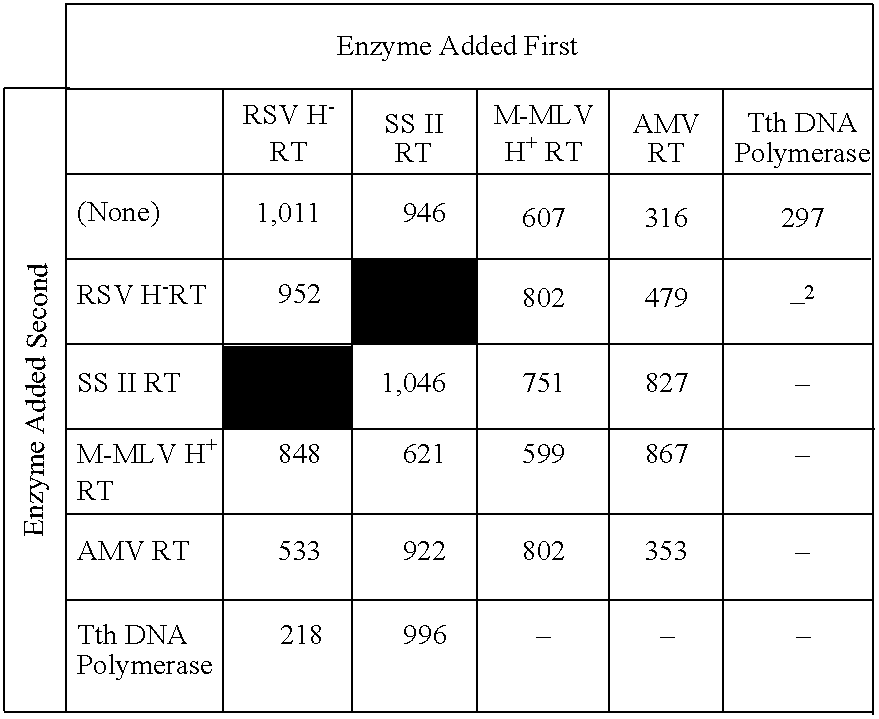
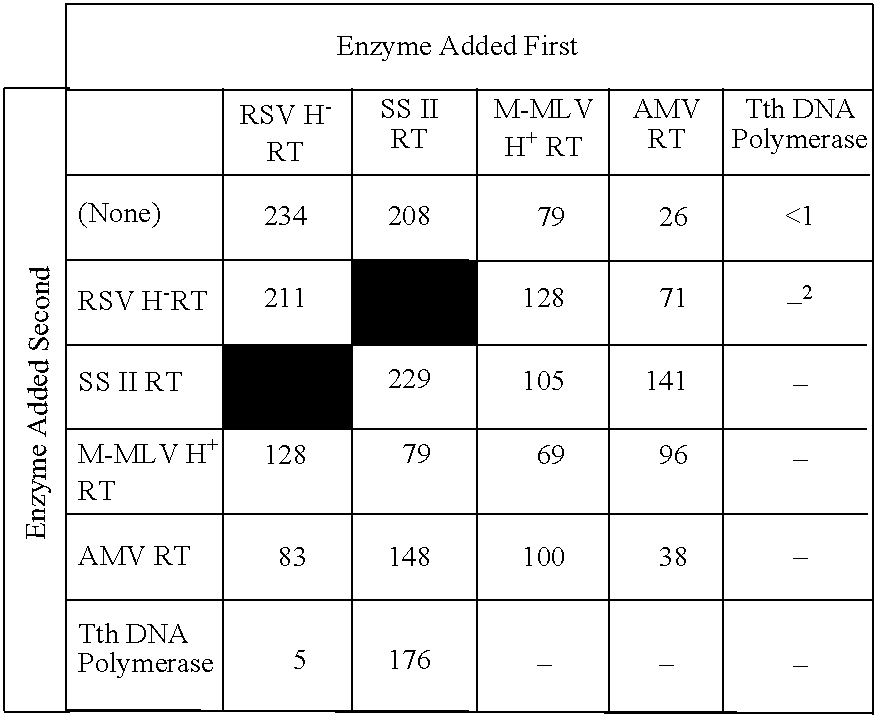
Recombinant method for making RSV reverse transcriptases and mutants thereof
A method of producing a Rous Sarcoma Virus reverse transcriptase (RSV RT) by expressing one orniore nucleic acid sequences encoding one or more subunits of RSV RT in a eukaryotic host cell and culturing the host cell under conditions sufficient to produce the recombinant RSV RT. The resulting RSV RT has a specific activity of between about 30,000 and 150,000 units per milligram and is suitable for methods including RT-polyinerase chain reaction (RT-PCR).
Owner:INVITROGEN
Phosponate nucleosides useful as active ingredients in pharmaceutical compositions for the treatment of viral infections, and intermediates for their production
InactiveUS20070185062A1Easy to tunePoor propertyBiocideAntibiotics chemistryViral Reverse TranscriptionMedicine
The present invention relates to novel phosponate nucleosides, more specifically to novel phosponalkoxy substituted nucleosides. The invention further relates to compounds having HIV (Human Immunodeficiency Virus) replication inhibiting properties and to compounds having antiviral activities with respect to other viruses. The invention also relates to methods for preparation of all such compounds and pharmaceutical compositions comprising them. The invention further relates to the use of said compounds as a medicine and in the manufacture of a medicament useful for the treatment of subjects suffering from HIV infection, as well as for treatment of other viral, retroviral or lentiviral infections and to the treatment of animals suffering from FIV, viral, retroviral or lentiviral infections.
Owner:K U LEUVEN RES & DEV
Grass carp reovirus (GCRV) reverse transcription polymerase chain reaction nucleic acid detection method
InactiveCN101629216AHigh sensitivityPracticalMicrobiological testing/measurementMicroorganism based processesViral Reverse TranscriptionTotal rna
The invention provides a grass carp reovirus (GCRV) reverse transcription polymerase chain reaction nucleic acid detection method, comprising the following steps: directly extracting the total RNA in the samples to be detected by adopting a RNA adsorption column centrifugal method; detecting the s10 gene segment of the structural protein VP 7 with the code of GCRV of the outer capsid by reverse transcription chain reaction. The sample to be detected comprises normal cells, cells infected by virus, normal fish tissues and tissues of infected fish. The detection method has the advantages that the total RNA can be extracted from the samples to be detected by the column centrifugal method and RT-PCR amplification can be carried out by only needing a small amount of tissue of the samples to be detected; the minimum detection sensitivity is 10<-6>ng, and the required time is only 4-5 hours; the detection method is simple and reliable, high in sensitivity and strong in specificity; and the result is prospective to be used for GCRV clinical quick diagnosis and entry-exit customs quarantine of fish fry.
Owner:WUHAN INST OF VIROLOGY CHINESE ACADEMY OF SCI
Detection kit and detection method of mandarin fish rhabdovirus
ActiveCN104032038BQuick checkExtensive use of disease surveillanceMicrobiological testing/measurementPositive controlAquatic animal
The invention belongs to the technical field of detection and diagnosis of aquatic animal pathogeny, and discloses a detection kit and detection method for siniperca chuatsi rhabdoviruses. The reverse transcription-polymerase chain reaction type rapid detection kit for the siniperca chuatsi rhabdoviruses is filled with a 5* reverse transcription buffer solution, reverse transcriptase, a random primer, 1.0 mL of 2*reaction mixed buffer solution, a preferably-designed sense primer, a preferably-designed reverse primer, Taq DNA polymerase, a positive control solution, a negative control solution and ddH2O. The novel reverse transcription-polymerase chain reaction type rapid detection kit and detection method for the siniperca chuatsi rhabdoviruses overcome shortcomings of an existing detection method for the siniperca chuatsi rhabdoviruses, are practicable when applied to clinical diagnosis of the siniperca chuatsi rhabdoviruses, have the advantages of rapidity, accuracy and specificity, meet requirements of the clinical diagnosis, and provide convenience for detection of the siniperca chuatsi rhabdoviruses.
Owner:GUANGZHOU JINSHUI ANIMAL HEALTH PROD
Compounds and methods for treating HIV
InactiveUS9163023B2Organic active ingredientsOrganic chemistryViral Reverse TranscriptionImmunodeficiency virus
Provided are compounds and pharmaceutically acceptable salts thereof, their pharmaceutical compositions, their methods of preparation, and their use for treating viral infections mediated by a member of the retrovirus family of viruses such as the Human Immunodeficiency Virus (HIV).
Owner:VIIV HEALTHCARE UK LTD
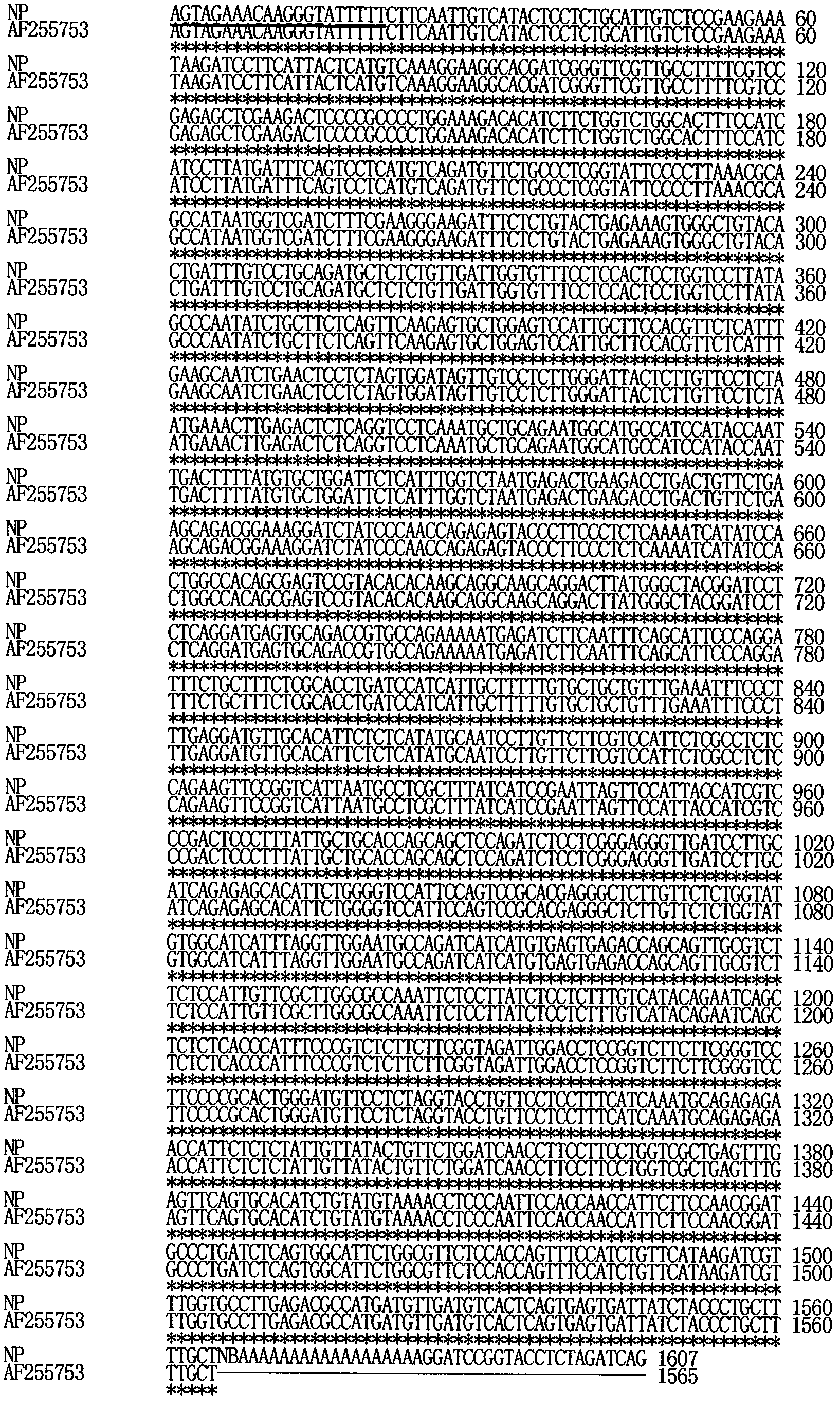
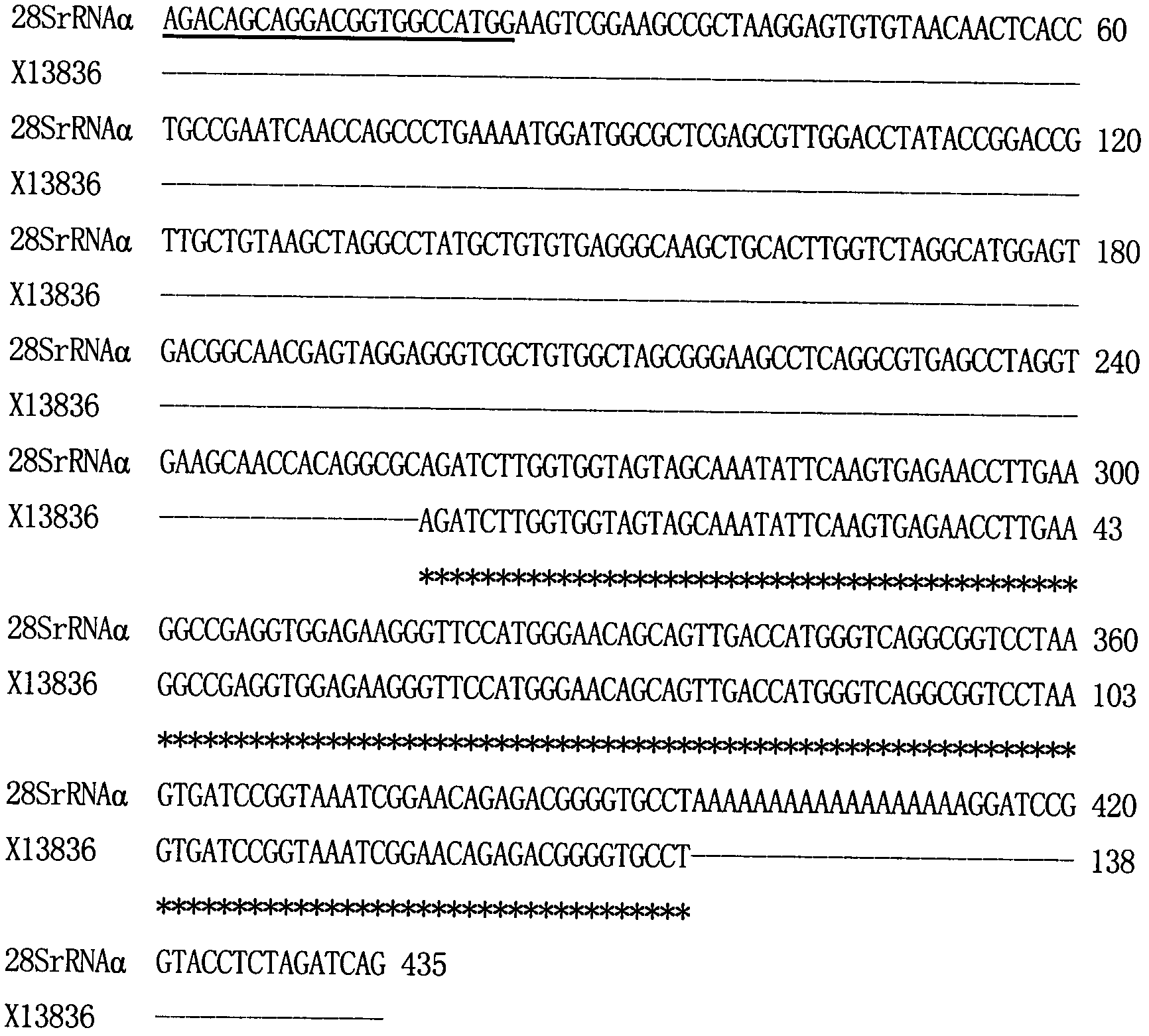
Method for confirming 3.-terminus sequence of virus RNA (Ribonucleic Acid) molecule
InactiveCN102618531AOmit designOmit featureMicrobiological testing/measurementDNA preparationCompound aViral Reverse Transcription
A method for confirming a 3.-terminus sequence of a virus RNA (Ribonucleic Acid) molecule comprises the following steps of: extraction of virus RNA, tail transfer reaction of the virus RNA, purification of tailing virus RNA, reverse transcription reaction of the tailing virus RNA, and polymerase chain reaction amplification of a product of the virus reverse transcription reaction. According to the invention, after tailing is performed on the virus RNA by the tail transfer reaction, the 3.-terminus sequence of the virus RNA molecule can be accurately confirmed by the reverse transcription reaction, thus the method is convenient and quick. As an Oligo dT primer or a modified Oligo dT primer is utilized to perform the reverse transcription reaction, the process of designing and compounding a specific primer according to a specific virus RNA molecule is eliminated, so that the period of an experiment is simplified greatly, and the experiment cost is saved. Simultaneously, the method can be applied to confirming other 3.-terminus sequences of the RNA molecule the 3.-terminus of which does not have a PolyA tail structure.
Owner:SHAANXI NORMAL UNIV
Nucleobase phosphonate analogs for antiviral treatment
InactiveUS20060252729A1Increasing cellular accumulation and retentionIncrease valueOrganic active ingredientsBiocideHigh concentrationAcquired immunodeficiency
The present invention provides novel compounds with activity against infectious viruses. The compounds of the invention may inhibit retroviral reverse transcriptases and thus inhibit the replication of the virus. They are useful for treating human patients infected with a human retrovirus, such as human immunodeficiency virus (strains of HIV-1 or HIV-2) or human T-cell leukemia viruses (HTLV-I or HTLV-II) which results in acquired immunodeficiency syndrome (AIDS) and / or related diseases. The present invention also relates generally to the accumulation or retention of therapeutic compounds inside cells. The invention is more particularly related to attaining high concentrations of active metabolite molecules in HIV infected cells. Intracellular targeting may be achieved by methods and compositions which allow accumulation or retention of biologically active agents inside cells. Such effective targeting may be applicable to a variety of therapeutic formulations and procedures.
Owner:GILEAD SCI INC
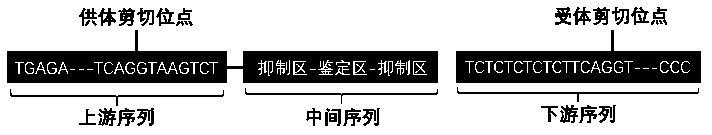
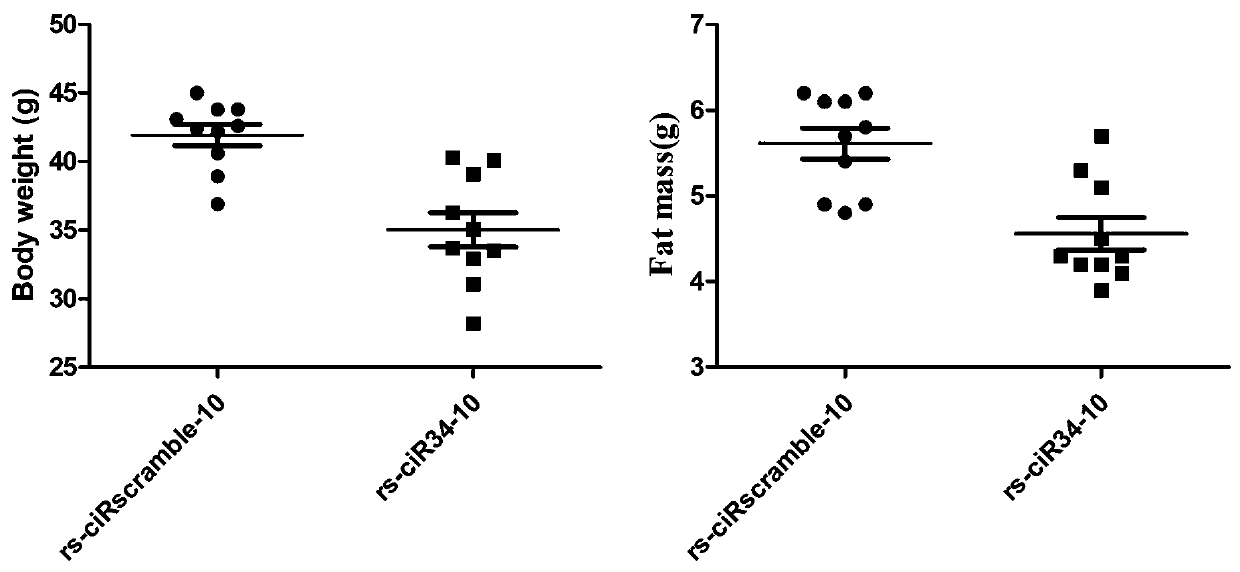
General expression framework of artificial circular RNA targeting to inhibit miRNA-34a, and expression method and application thereof
InactiveCN109554368AOrganic active ingredientsMetabolism disorderFatty liverSecondary hyperlipidemia
The invention relates to a general expression framework of artificial circular RNA targeting to inhibit miRNA-34a, and an expression method and an application thereof. The general expression frameworkconsists of three parts: an upstream sequence, an intermediate sequence and a downstream sequence. The general expression framework of the artificial circular RNA targeting to inhibit miRNA-34a can be mediated in cells by plasmids or viral tools to express artificial circular RNA molecules targeting to inhibit miRNA-34a, and has the ability to inhibit the biological function of miRNA-34a-5p in cells. The invention also relates to the general expression framework and the application of eukaryotic expression plasmids, lentiviruses, adenoviruses, adeno-associated viruses and retroviruses carrying the expression framework in preparation of drugs for obesity, fatty liver, hyperlipidemia and diabetes.
Owner:浙江自贸区锐赛生物医药科技有限公司
Compositions and methods for RT-PCR
ActiveUS9353409B2Rapid and efficient amplificationImprove reliabilityMicrobiological testing/measurementFermentationBiologyNucleoside triphosphate
The present invention relates to methods and compositions having trehalose and DNA polymerase for facilitating the rapid and efficient amplification of nucleic acid molecules and the detection and quantitation of RNA molecules, and for increasing the detection sensitivity and reliability through generation of secure cDNA molecules prior to gene-specific primer dependent amplification. The reagent mixture comprises a ready to use reagent solution, wherein the solution comprises: (a) trehalose in a concentration between about 5% and about 35%; (b) a viral reverse transcriptase; and (c) at least one DNA polymerases, in a buffer suitable for use in a reverse transcription reaction, wherein the buffer comprises a co-factor metal ion and nucleoside triphosphates.
Owner:LEE JUN EUIHUM
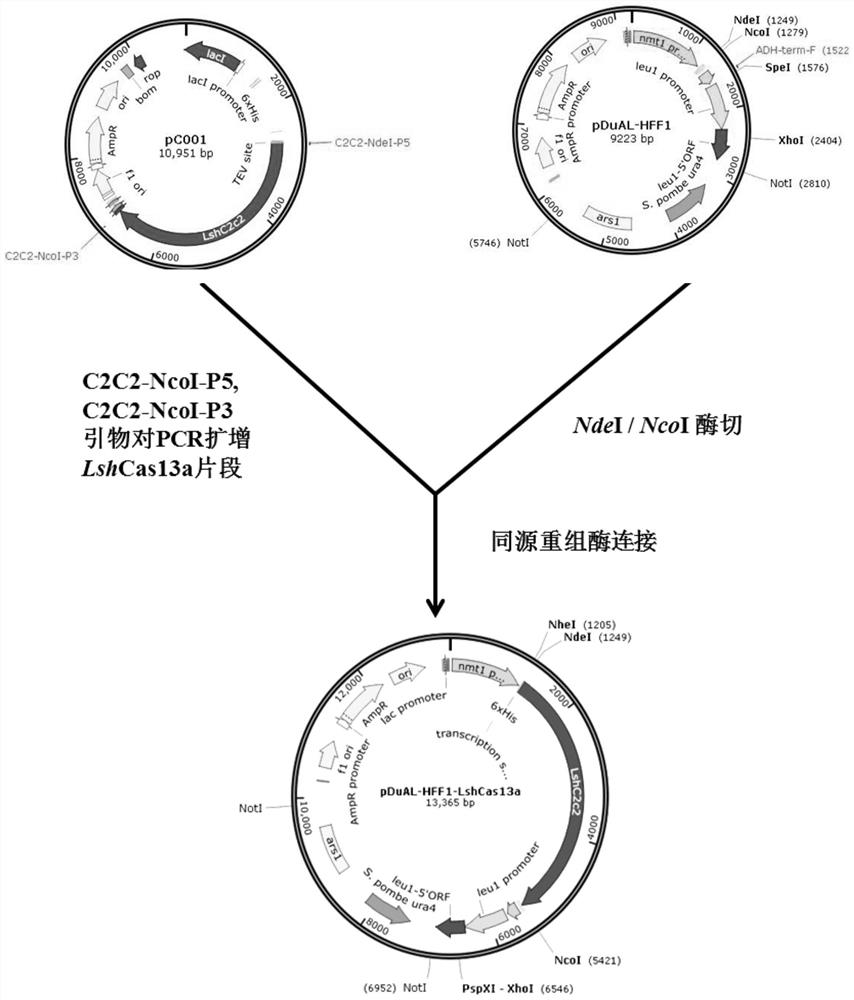
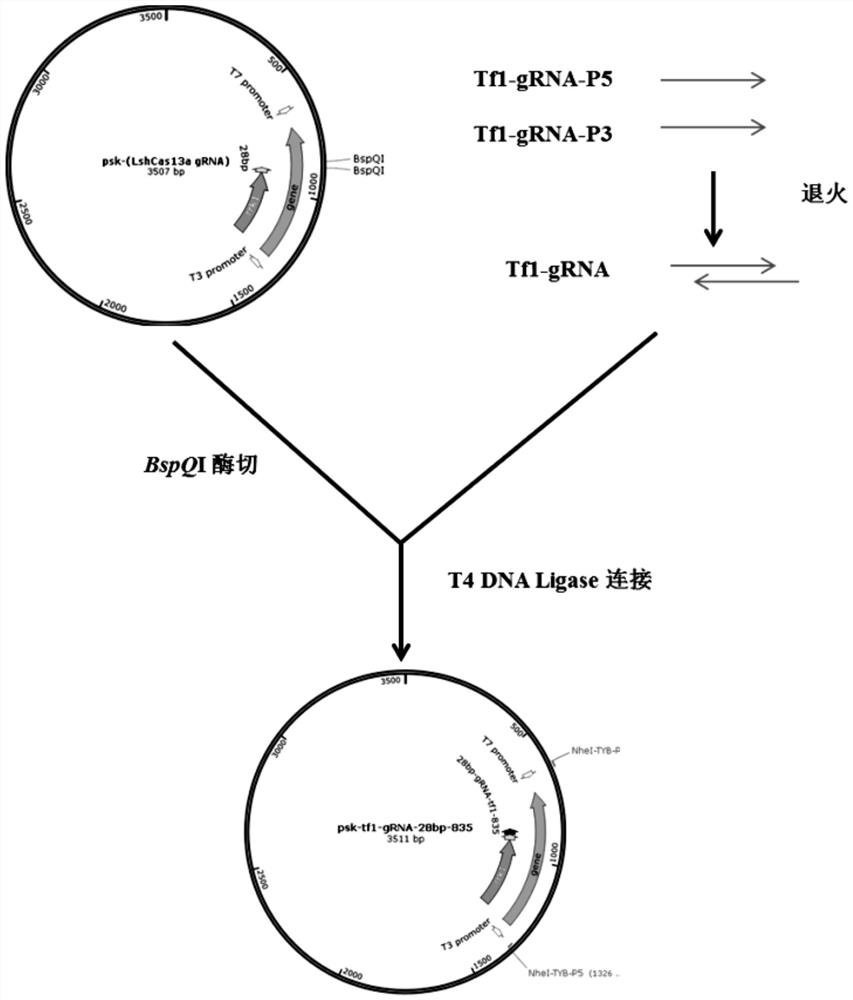
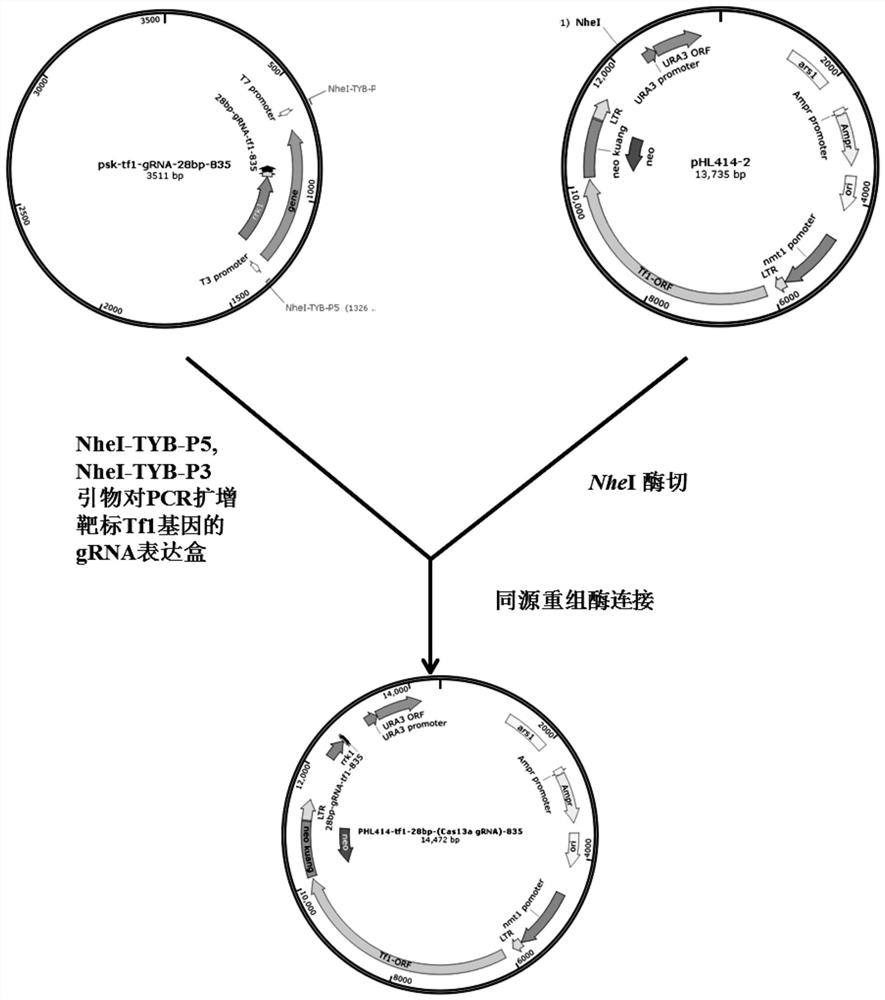
Technology for intervening and blocking virus reverse transcription transposition based on CRISPR-Cas13a
ActiveCN113528515AStable introduction of DNAVector-based foreign material introductionViral Reverse TranscriptionBioinformatics
The invention provides a technology for intervening and blocking reverse transcription transposition of a reverse transcription organism based on CRISPR-Cas13a. The invention discloses a technology for intervening and blocking reverse transcription transposition of the reverse transcription organism based on CRISPR-Cas13a, a system for blocking reverse transcription transposition of the reverse transcription organism in eukaryotic cells and application of the system. In the invention, research and demonstration are carried out on the basis of the model system of the retrovirus Tf1. According to the technical scheme, Cas13a is used for inhibiting reverse transcription transposition for the first time, and the reverse transcription transposition blocking efficiency of Cas13a is ideal.
Owner:CAS CENT FOR EXCELLENCE IN MOLECULAR PLANT SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com