Caprine herpesvirus I vaccine strain and application thereof
A technology of herpes virus and vaccine strains, applied in the direction of antiviral agents, viruses/phages, medical preparations containing active ingredients, etc., to achieve strong pathogenicity, reduce morbidity, and stabilize biological characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
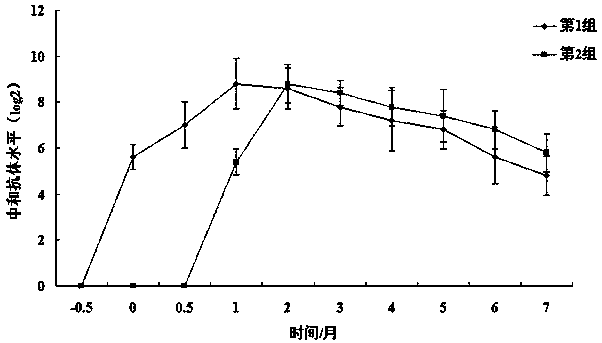
Image
Examples
Embodiment 1
[0018] Example 1 Isolation and Identification of Goat Herpesvirus Type Ⅰ JSHA1405 Strain
[0019] 1. In situ samples and cells
[0020] In 2014, large-scale goat farms in Hai'an County, Jiangsu Province and other places in my country successively appeared diseases with respiratory symptoms as the main manifestations, manifested as depression, panting, coughing, serous fluid or thick nasal fluid. The nasal swabs of the affected goats were collected and stored at -80°C. Bovine kidney passage cells (MDBK) were purchased from China Veterinary Drug Administration.
[0021] 2. Virus isolation and identification
[0022] 2.1 Isolation and purification of virus
[0023] The nasal swab samples collected from the diseased goats were sterilized by filtration through a 0.22 μm filter membrane, inoculated in MDBK monolayer cell culture, and incubated at 37°C, 5% CO 2 The cells were cultured in the dark in the incubator, and the cytopathic changes (CPE) were observed every day. When bl...
Embodiment 2
[0037] The immune effect test of embodiment 2 goat herpes virus type Ⅰ inactivated vaccine (JSHA1405 strain)
[0038] 1. Poison species
[0039] The virus seed used for making seedlings is goat herpesvirus type Ⅰ JSHA1405 strain, which has been preserved in the China Center for Type Culture Collection. The preservation information is as follows:
[0040] Taxonomic name: goat herpesvirus type Ⅰ JSHA1405 strain
[0041] Caprine Herpesvirus 1JSHA1405;
[0042] Address: Wuhan University, Wuhan, China, zip code 430072;
[0043] Deposit number: CCTCC NO: V201745;
[0044] Deposit date: October 24, 2017.
[0045] 2. Preparation of poisonous seeds
[0046] Cultivate MDBK cells until the confluence reaches 70%, remove the culture medium, inoculate goat herpesvirus type I JSHA1405 strain, add cell maintenance medium (DMEM medium containing 2% fetal bovine serum), and incubate at 37°C, 5% CO 2 Culture in the dark in the cell culture box, collect the cell culture 48 hours after inoc...
Embodiment 3
[0059] Example 3 Safety Test of Goat Herpes Virus Type Ⅰ Inactivated Vaccine (JSHA1405 Strain)
[0060] 1. Experimental vaccines
[0061] According to the method in Example 2, 3 batches of goat herpes virus type I inactivated vaccines were prepared in the laboratory, and the batch numbers were: SZ-001, SZ-002, SZ-003, 500 heads / bottle, 2-8 Store at ℃.
[0062] 2. Experimental animals
[0063] Healthy pregnant ewes and 2-3-month-old healthy lambs negative for CpHV-1 antigen and antibody were purchased from a goat farm in Jurong City, Jiangsu Province.
[0064] 3. Lamb safety test
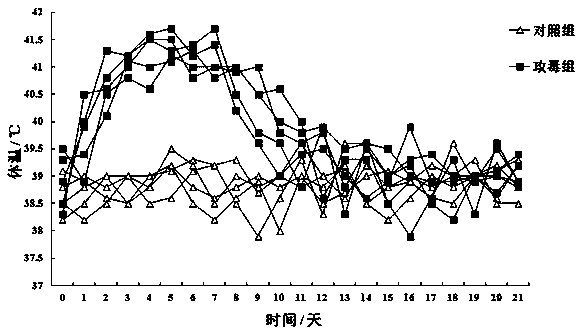
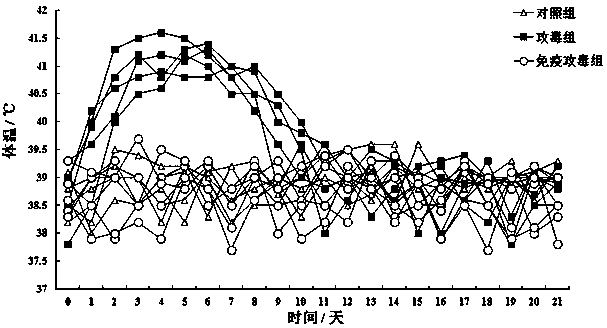
[0065] 3.1 Grouping and scheme: 50 lambs aged 2-3 months were randomly divided into 4 groups (single dose group, double dose group, single dose repeat group and control group), of which single dose group, double dose group 1. There were 15 rats in each group in the single-dose repeated group, which were divided into 3 different procedures for immunization, and 5 rats in the control group. Single...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com