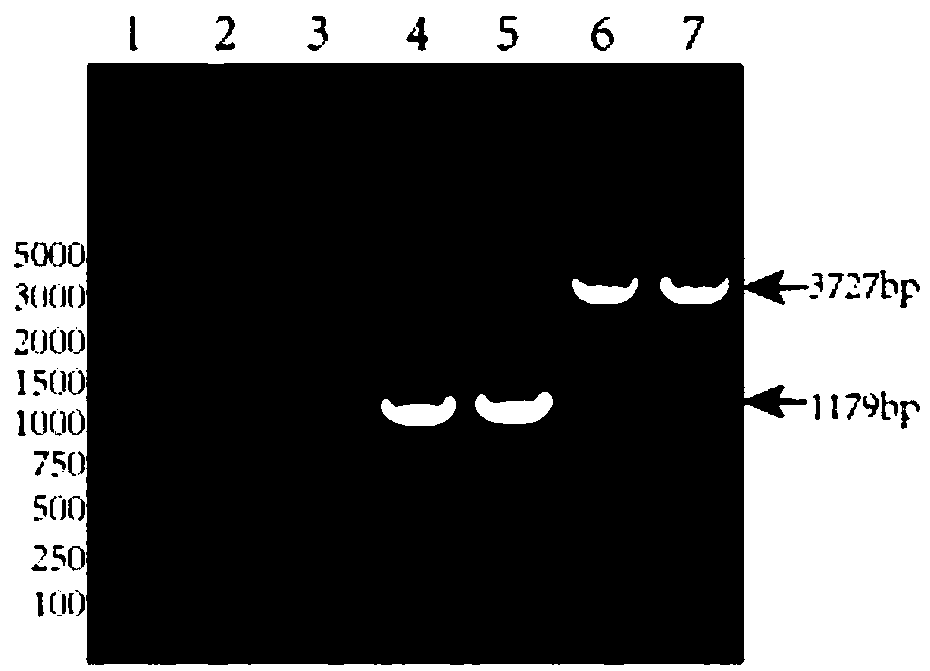
Patents
Literature
40 results about "Orf virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Orf is an exanthemous disease caused by a parapox virus and occurring primarily in sheep and goats. It is also known as contagious pustular dermatitis, infectious labial dermatitis, ecthyma contagiosum, thistle disease and scabby mouth. Orf virus is zoonotic—it can also infect humans.
Preparation method of permanent cell line for multiplying orf virus
ActiveCN102533660AStable proliferationMicroorganism based processesForeign genetic material cellsTesticleAttenuated vaccine
The invention relates to a preparation method of a permanent cell line for multiplying an orf virus. The aim that the virus can be stably multiplied in a large amount is achieved by inoculating a calf testicular cell line to the orf virus and a stable cell environment and a standardized culture system for developing and producing an attenuated vaccine of the orf virus are provided. The preparation method comprises the following steps of: firstly, acquiring, separating and culturing a calf testicular cell; secondly, permanently establishing the calf testicular cell; and thirdly, carrying out multiplication culture on the orf virus in the cell line.
Owner:NORTHWEST A & F UNIV
Cell line used for separation culture and multiplication of Orf virus (ORFV) as well as preparation method and application of cell line
ActiveCN103952377AAvoid the risk of carrying other pathogensQuality improvementMicroorganism based processesVertebrate cellsImpetigo contagiosaTiter
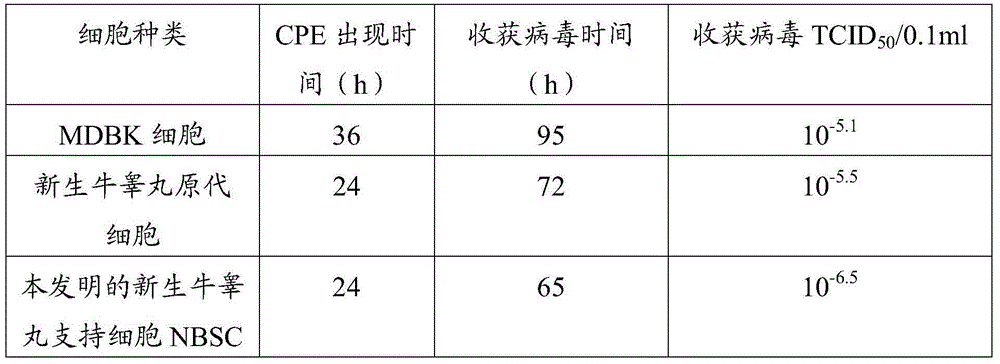
The invention discloses a cell line used for separation culture and multiplication of an Orf virus (ORFV) as well as a preparation method and application of the cell line. The cell line disclosed by the invention is a newly born bovine testis supported cell line obtained by separating and purifying newly born bovine testis primary culture cells; research results show that the cell line is more sensitive to inoculation of contagious ovine ecthyma virus, virus titer multiplied by utilizing the cell line is about 1 virus titer higher than that of newly born bovine testis primary culture cells and 1.9 virus titer higher than that of a madin-darby bovine kidney (MDBK) cell line. ORFV is separated by utilizing the cell line, uniformity and stability of virus can be guaranteed, and a risk that the separated viruses carry other pathogens as the primary culture cells are utilized for multiple times can be prevented; besides, the passage cell line is utilized for preparing a vaccine, a production process of the vaccine can be simplified, a production cycle is shortened, production cost is reduced, and stable quality of the prepared vaccine is also guaranteed, so that the cell line has a certain practical significance on large-scale production of the vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Goatpox virus-orf virus combined cell attenuated vaccine and its preparation method and use
ActiveCN104758928AGuaranteed immune protectionSave solutionAntiviralsMacromolecular non-active ingredientsActive componentAttenuated vaccine
The invention discloses a goatpox virus-orf virus combined cell attenuated vaccine and its preparation method and use. The goatpox virus-orf virus combined cell attenuated vaccine contains an active component which comprises goatpox and contagious pustular dermatitis virus cell passage-attenuated viruses or passaged viruses separated by the inventor and subjected to passage attenuation culture, wherein the goatpox virus cell passage-attenuated virus is a Goatpox Virus HN-XY2010F43 strain and has a preservation number of CCTCC NO: V201503 and the contagious pustular dermatitis virus cell passage-attenuated virus is an Orf Virus HB-TS09F65 strain and has a preservation number of CCTCC NO: V201406. A toxin counteracting protection test result shows that the goatpox virus-orf virus combined cell attenuated vaccine is safe and can produce an effective immunization protection function. The goatpox virus-orf virus combined cell attenuated vaccine and its preparation method have important practical meanings for development of safe and efficient attenuated vaccines and large-scale production of the goatpox virus-orf virus combined cell attenuated vaccine for preventing and controlling goatpox and orf from the disclosed attenuated strain.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Goatpox, sheeppox and contagious ecthyma triple cell attenuated vaccine and preparation method and application thereof
ActiveCN106075429AAvoid the risk of carrying other pathogensQuality improvementViral antigen ingredientsAntiviralsAttenuated vaccineSheeppox
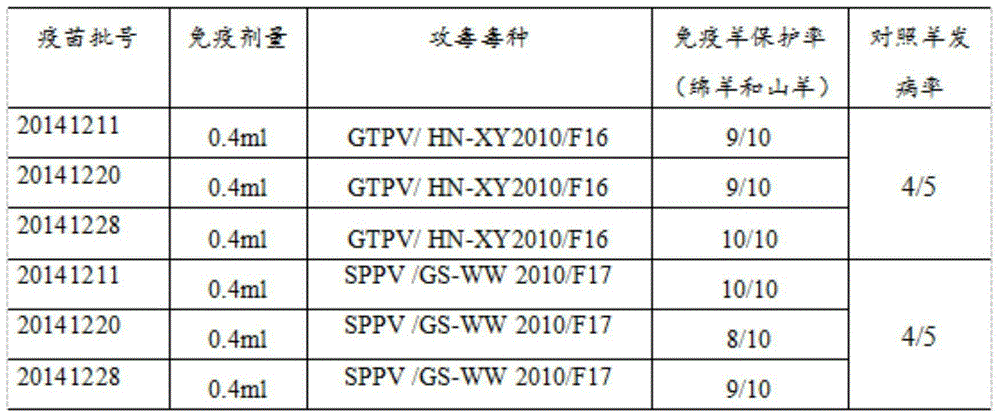
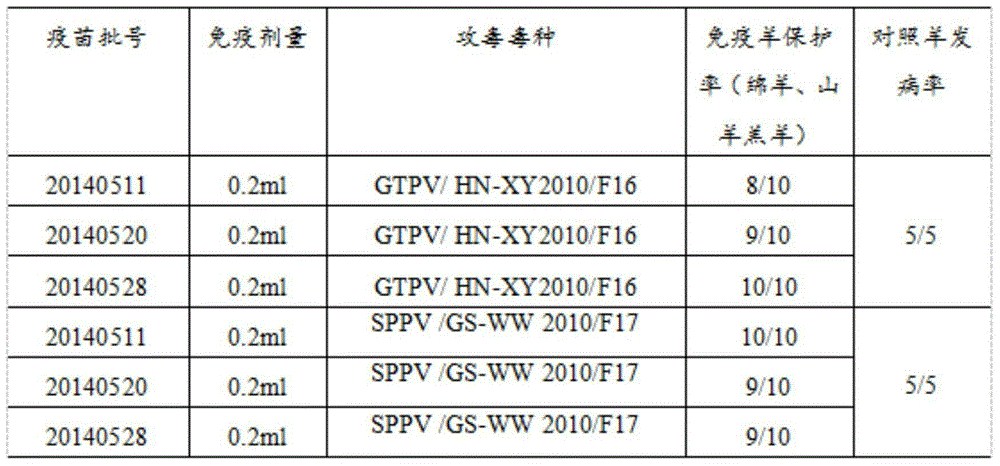
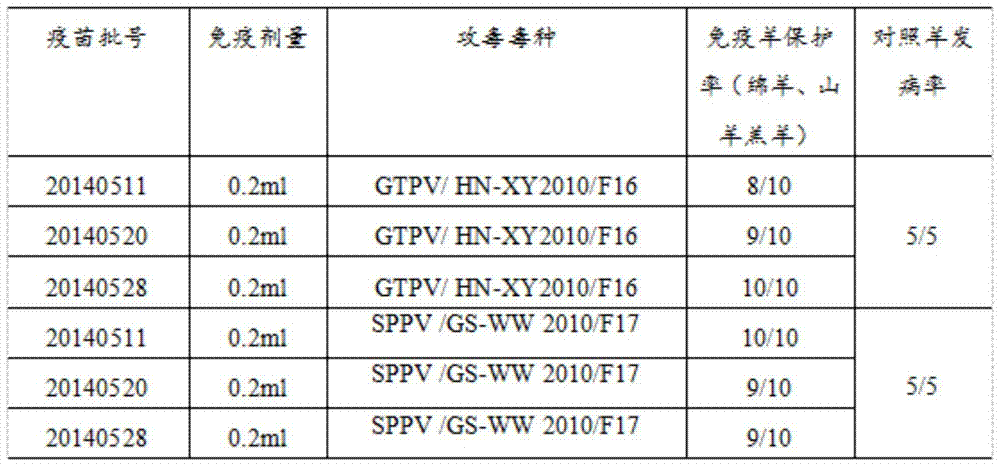
The invention discloses a goatpox, sheeppox and contagious ecthyma triple cell attenuated vaccine and a preparation method and application thereof. The effective components of the vaccine are separated by an inventor automatically, and after attenuation culture is conducted, goatpox virus, sheeppox virus and Orf virus cell passage attenuated viruses or passage viruses thereof are obtained, wherein a goatpox virus is a Goatpox Virus HN-XY2010F43 strain, a sheeppox virus is a Sheeppox Virus GS-WW 2010 F44 strain, and an Orf virus is an Orf Virus HB-TS09F65 strain. It is shown through attacking protection test results that the prepared goatpox, sheeppox and contagious ecthyma triple cell attenuated vaccine is safe and capable of producing an effective immune protection force, and important practical significance in developing safe and efficient goatpox, sheeppox and contagious ecthyma triple cell attenuated vaccine and preventing and controlling goatpoxes and ecthyma is achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Orf virus (ORFV) protein ORFV059 monoclonal antibody hybridoma G3 and monoclonal antibody
InactiveCN102533666AImprove featuresHigh sensitivityImmunoglobulins against virusesTissue cultureImmunofluorescent stainWestern blot
The invention discloses an orf virus (ORFV) protein ORFV059 monoclonal antibody hybridoma G3 and a monoclonal antibody. The ORFV059 monoclonal antibody hybridoma G3 was collected in China General Microbiological Culture Collection Center (CGMCC) on November 4, 2011, wherein the collection number is CGMCC No.5425. The recombinant ORFV059 protein coats an enzyme-linked immuno sorbent assay (ELISA) plate; activity of a purified antibody is detected by an ELISA method; and the purified ORFV059 protein is subjected to Western-blot by the purified antibody, which proves that the antibody can identify the ORFV059 protein. The purified antibody is used for performing immunofluorescent staining on the ORFV059 and immunohistochemical staining on the orf tissue, which proves that the purified antibody can identify the natural ORFV059 protein. The monoclonal antibody secreted by the hybridoma cell lays basis for further development of ORFV059 function and a diagnosis reagent for developing the ORFV059 and provides a powerful tool for clinical diagnosis on the orf and confirmation of a therapeutic target.
Owner:SOUTHERN MEDICAL UNIVERSITY
Real-time fluorescent quantitative PCR (polymerase chain reaction) detecting reagent kit for orf virus
InactiveCN102676694AControl spreadQuality assuranceMicrobiological testing/measurementMicroorganism based processesNucleotideNucleotide sequencing
The invention discloses a real-time fluorescent quantitative PCR (polymerase chain reaction) detecting reagent kit for an orf virus and belongs to the technical field of viral molecular biology detection. The real-time fluorescent quantitative PCR detecting reagent kit for the orf virus comprises a pair of primers of nucleotide sequences shown in SEQ ID NO:1-2 and a TaqMan probe of a nucleotide sequence shown in SEQ ID NO:3. The detecting reagent kit can quickly and accurately detect the orf virus from samples and is high in detecting sensitivity and simple and convenient to sample, detecting efficiency is greatly improved, pollution can be effectively prevented, and the detecting reagent kit is of practical significance in the field of inspection and quarantine of import and export animals.
Owner:GUANGZHOU BONIZZI BIOTECH CO LTD
Goatpox and sheep pox bivalent cell attenuated vaccine as well as preparation method and application thereof
ActiveCN104800842AEffective immune protectionR&D safetyAntiviralsAntibody medical ingredientsAdditive ingredientAttenuated vaccine
The invention provides a goatpox and sheep pox bivalent cell attenuated vaccine. The goatpox and sheep pox bivalent cell attenuated vaccine comprises effective ingredients, namely, a goatpox virus cell passage attenuation virus and a sheep pox virus cell passage attenuation virus, wherein the goatpox virus cell passage attenuation virus is a goatpox virus cell passage attenuation vaccine strain Goatpox Virus HN-XY2010F43 strain CCTCC V201503, and the sheep pox virus cell passage attenuation virus is a sheeppox virus cell passage attenuation vaccine strain Sheeppox Virus GS-WW2010F44 strain CCTCC V201504. The goatpox and orf virus bivalent cell attenuated vaccine is safe, can have the effective immune protection force and has the important practical significance in development of safe and efficient attenuated vaccines as well as prevention and control on goatpox and sheeppox through large-scale production of goatpox and sheep pox virus bivalent cell attenuated vaccines by the aid of the attenuation virus strains.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Double PCR detection primer, double PCR detection reagent kit and double PCR detection method for identification of orf virus and capripoxvirus
ActiveCN106086232AStrong specificityIncreased sensitivityMicrobiological testing/measurementDNA/RNA fragmentationCapripoxvirusReagent
The invention discloses a double PCR detection primer, a double PCR detection reagent kit and a double PCR detection method for identification of orf virus and capripoxvirus. The method provides a rapid detection method for diagnosis of the orf virus and capripoxvirus. Particularly, the PCR primer for detection of the capripoxvirus relates to all the members of capripoxvirus, including goat capripoxvirus, sheep capripoxvirus, and lumpy skin disease virus, so that the detection method has the characteristics of highlight integration and high-throughput performance, etc., and can provide technical support and help for diagnosis of the orf virus and capripoxvirus, and investigation, prevention and treatment of epidemic virus. The method has the advantages of high specificity, high sensitivity and time saving and labor saving.
Owner:INST OF ANIMAL HEALTH GUANGDONG ACADEMY OF AGRI SCI
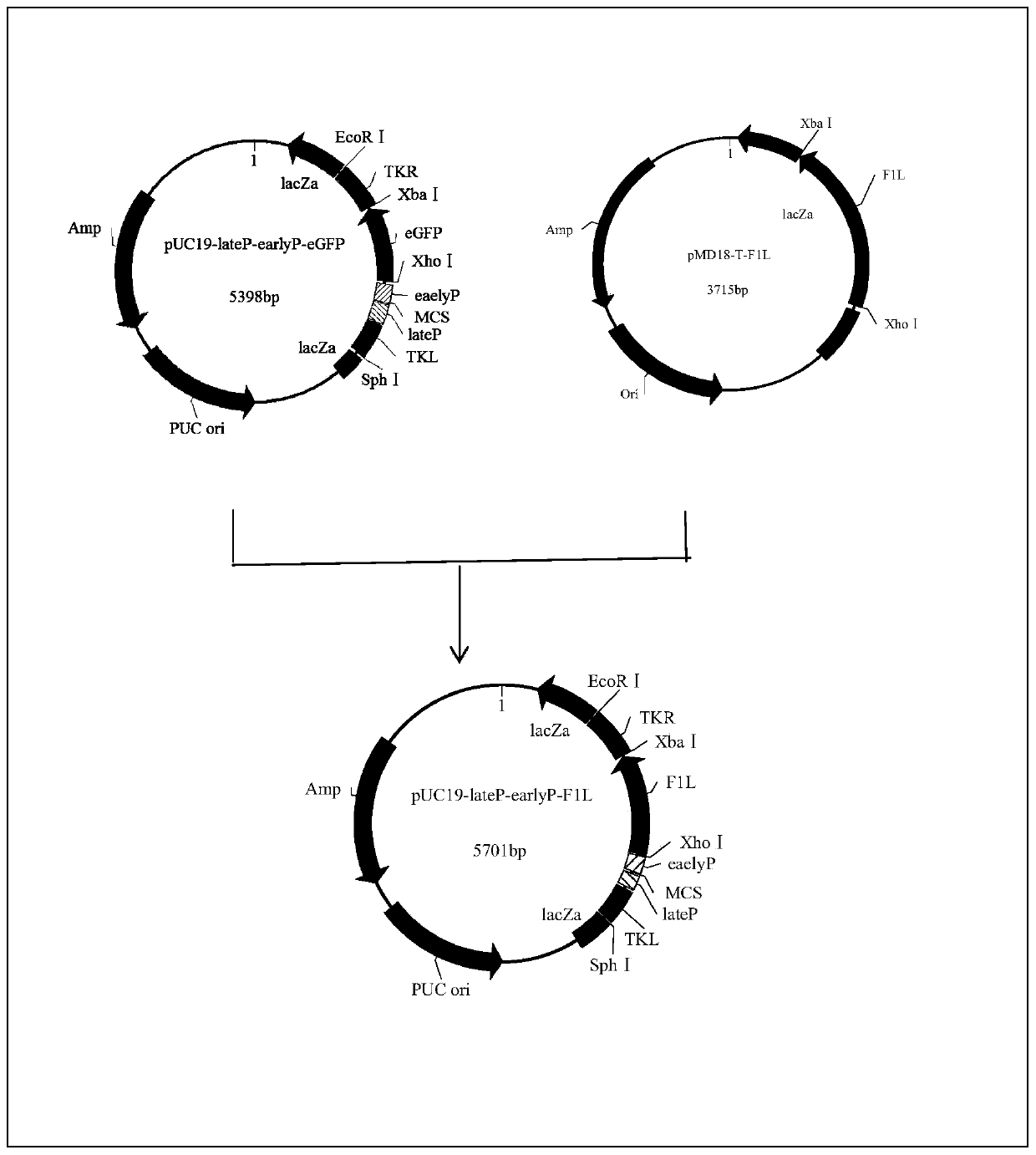
Selection marker-free recombinant goat-pox virus for expression of Orf virus F1L protein and construction method thereof
InactiveCN110724674AHigh expressionContinuous and efficient expressionViral antigen ingredientsMicroorganism based processesEngineeringRecombinant virus vaccine
Through homologous recombination and by innovative utilization of plaque and reverse plaque screening technologies, the selection marker-free recombinant virus, especially the rGTPV-F1L recombinant goat-pox virus is finally obtained. The method is simple and effective, is easy to operate, provides the selection marker-free recombinant virus for the development of a recombinant virus vaccine, provides new ideas and measures for the researches on a novel ORF vaccine, and has broad application prospects.
Owner:CHONGQING ACAD OF ANIMAL SCI
Recombinant contagious ecthyma oncolytic virus and preparation method and application thereof
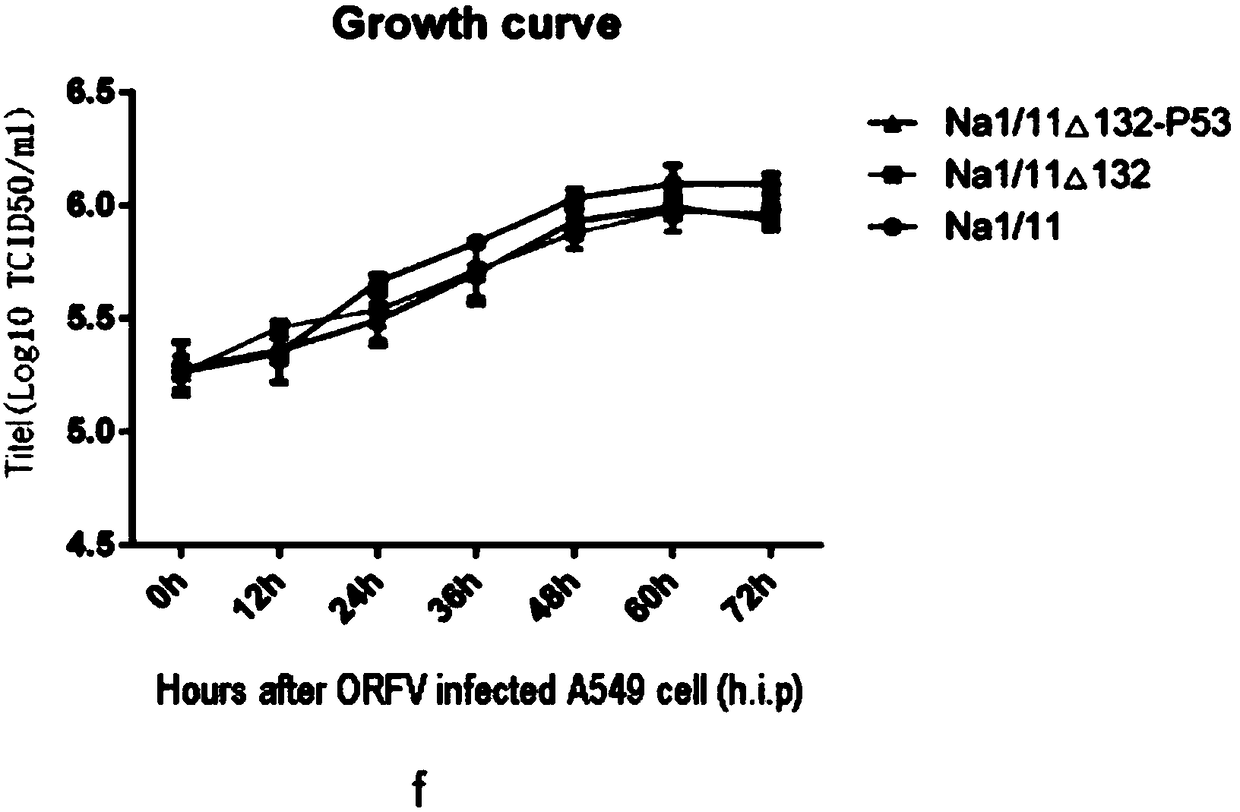
ActiveCN108220251AEasy to operateShorten screening timePeptide/protein ingredientsP53 proteinFluorescenceShuttle plasmid
The invention discloses ORFV (Orf virus) oncolytic virus and a preparation method and application thereof. The recombinant contagious ecthyma oncolytic virus preparation method disclosed by the invention comprises the following steps: (1) overlap PCR amplifying gene p53 of protein shown in claim 7; (2) connecting to a pSPV-EGFP carrier, inserting into the p53 gene to obtain recombinant plasmid p53-pSPV-EGFP and then inserting into the left side and the right side of ORFV132 gene to construct shuttle plasmid ORFV132LF-p53-pSPV-EGFP-ORFV132RF; (3) converting host bacteria TOP10 to obtain positive recombinant bacteria; (4) performing transfection on OFTu cells and performing fluorescent identification on fusion expression of the p53 and the EGFP to prepare the purified recombinant contagiousecthyma oncolytic virus. The prepared recombinant contagious ecthyma oncolytic virus can be duplicated and multiplicated in OFTu and varieties of tumor cells and can effectively inhibit growth of varieties of tumor cell. The recombinant contagious ecthyma oncolytic virus disclosed by the invention can be observed in a microscope; thus, easiness in operation is achieved, convenience and quickness are achieved, and screening time is greatly shortened.
Owner:SOUTHERN MEDICAL UNIVERSITY
Passage culture type sheep testis cell and passage domesticating culture method, and special culture system and application thereof
ActiveCN107058212AExtended passage timesSuitable for mass productionMicroorganism based processesArtificial cell constructsPhysiologyTGE VACCINE
The invention discloses a passage culture type sheep testis cell and a passage domesticating culture method, and a special culture system and application thereof, and provides a passage culture type sheep testis cell LT-1 (CGMCC No.12988) and a method for obtaining the cell by domesticating culture. The method has the advantages that optimized cell culture liquid is used for performing passage domesticating culture on a sheep testis primary cell (LT), the passage times of the sheep testis primary cell can be increased, and the method is suitable for batched production; the sheep orf virus is subject to multiplication culture by the cell; proofed by results, the sheep orf virus can be continuously subject to passage culture for three generations, the obvious lesion is produced, the content of virus reaches 107.5TCID50 / mL, and the method can be applied to the multiplication culture and vaccine preparation of the sheep orf virus.
Owner:JINYUBAOLING BIO PHARMA CO LTD
Taqman fluorescence quantitative PCR kit and method for simultaneously detecting eight common sheep viruses and bacteria
InactiveCN108504780AQuick checkSensitive detectionMicrobiological testing/measurementMicroorganism based processesEscherichia coliFluorescence
The invention discloses a Taqman fluorescence quantitative PCR kit and method for simultaneously detecting eight common sheep viruses and bacteria, and relates to the technical field of molecular biology. The eight common sheep viruses and bacteria include peste des petits ruminants virus (PPRV), orf virus (ORFV), goatpox virus (GTPV), chlamydophila abortus (C.a), staphylococcus aureus (S.a), escherichia coli (E.coli), streptococcus (Stre.) and salmonella (Salm.). PCR reaction liquid comprises eight groups of primers and eight groups of Taqman probes. The kit can rapidly and effectively detect8 sheep pathogens simultaneously, the detection method is accurate, the specificity and the sensibility are high, the stability is good, and it can be achieved that to-be-detected pathogens is rapidly diagnosed and effectively detected.
Owner:NORTHWEST A & F UNIV
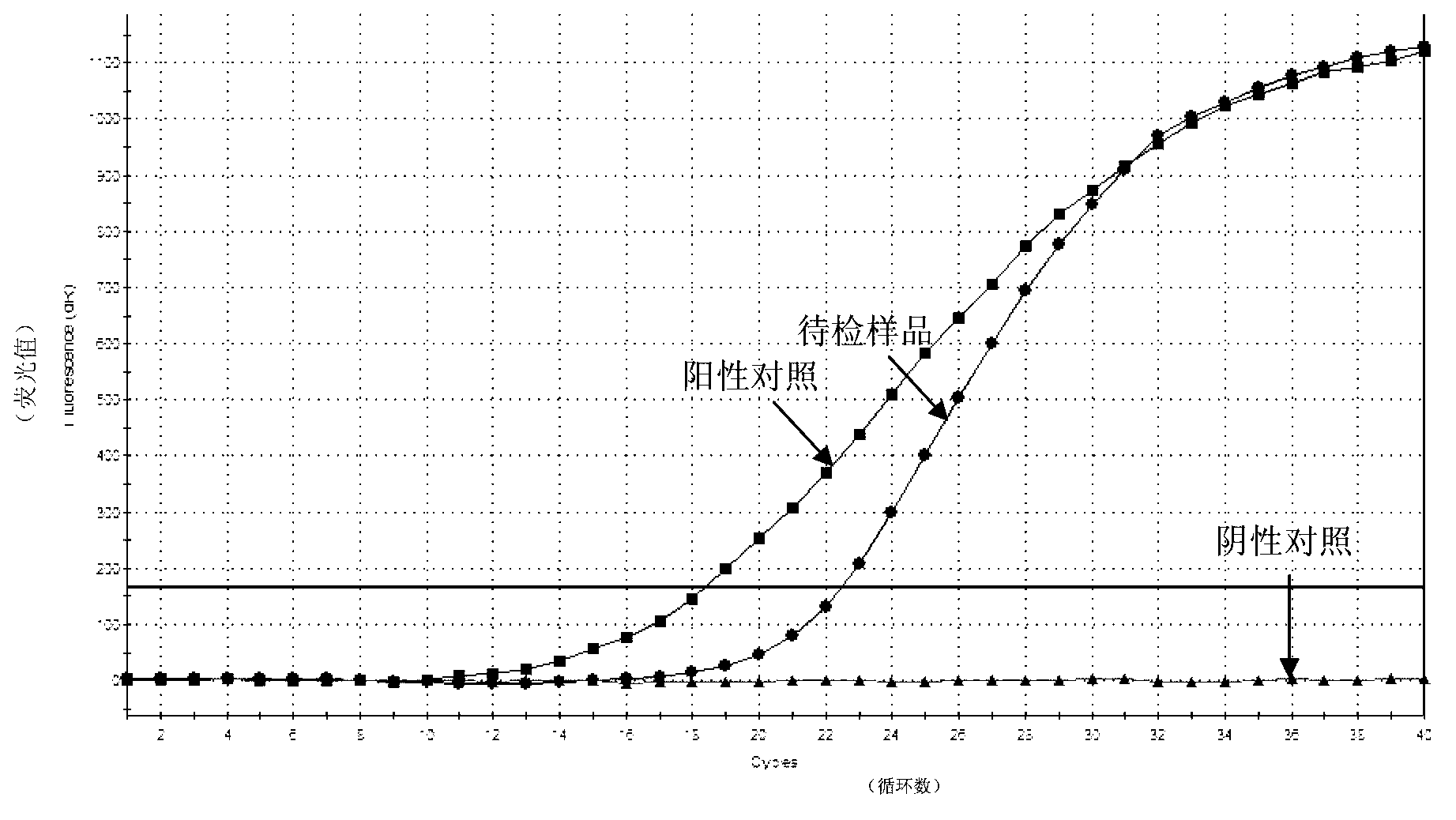
Kit for detecting orf virus of sheep and detection method thereof
ActiveCN103255234AStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceA-DNAVirology
The invention provides a detection method for detecting the orf virus of sheep. Real-time fluorescent quantitative PCR (polymerase chain reaction) detection is performed on a DNA (deoxyribonucleic acid) template of a sample by designing a pair of primers and a probe. The invention further provides a kit for detecting the orf virus of the sheep by utilizing the detection method. The real-time quantitative method which is established against H2L genes in a conserved region of the ORFV (orf virus) is high in specificity and good in stability. The kit for detecting the orf virus of the sheep, provided by the invention, is simple and convenient to operate, a user only needs to add DNA of the sample to be detected into a reaction tube, detection results can be obtained according to an amplification curve, the operation is time-saving and labor-saving, the cost is lower, and the time is shortened from original 3-4 hours to 1-2 hours; and furthermore, the sensitivity is higher than that of the ordinary PCR, and the detection method and the kit can be used for detecting the low content of the orf virus of the sheep, can be suitable for all levels of prevention and control units, basic-level veterinary stations, large and medium-sized breeding farms and the like in China, and has better application prospects.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
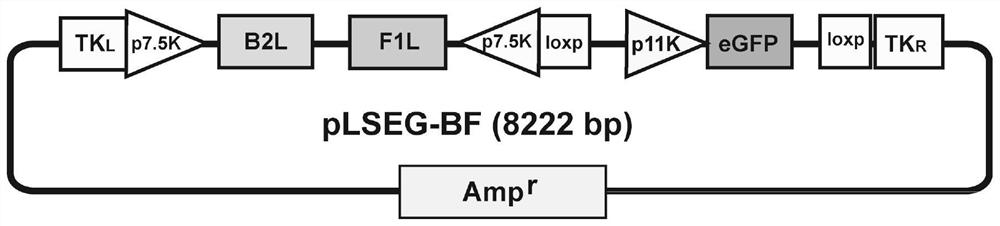
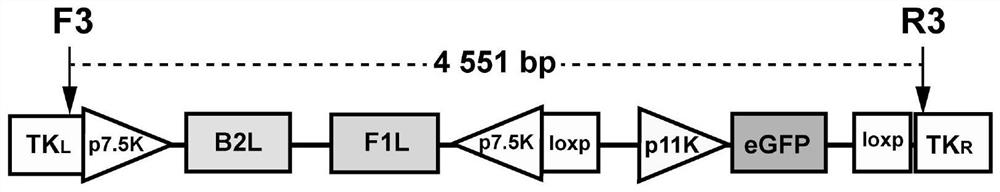
Recombinant goatpox virus capable of carrying out coexpression on orf virus B2L and F1L protein
The invention provides a recombination transfer vector used for constructing a recombinant goatpox virus. The transfer vector mainly comprises the left arm and the right arm of a TK gene, two reversep7.5K promoters, an orf virus B2L gene and F1L gene independently regulated and controlled by the two reverse p7.5K promoters, a p11K promoter, an enhanced green fluorescence protein (eGFP) gene and two forward Loxp sequences, wherein the transfer vector and the goatpox virus generate homologous recombination in goat testicular cells so as to obtain the recombinant goatpox virus capable of expressing the eGFP, under the function of Cre enzyme, the eGFP gene and the p11K prompter are specifically knocked out, and therefore, the potential recombinant goatpox virus capable of carrying out the coexpression on the orf virus B2L and F1L protein is obtained.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT
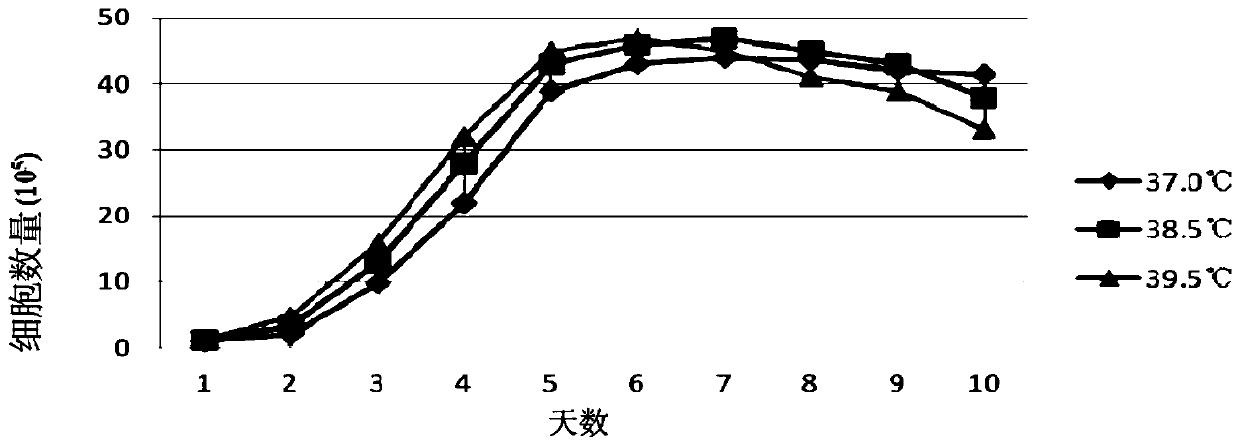
Sheep testis immortalized cell domestication method suitable for full suspension culture
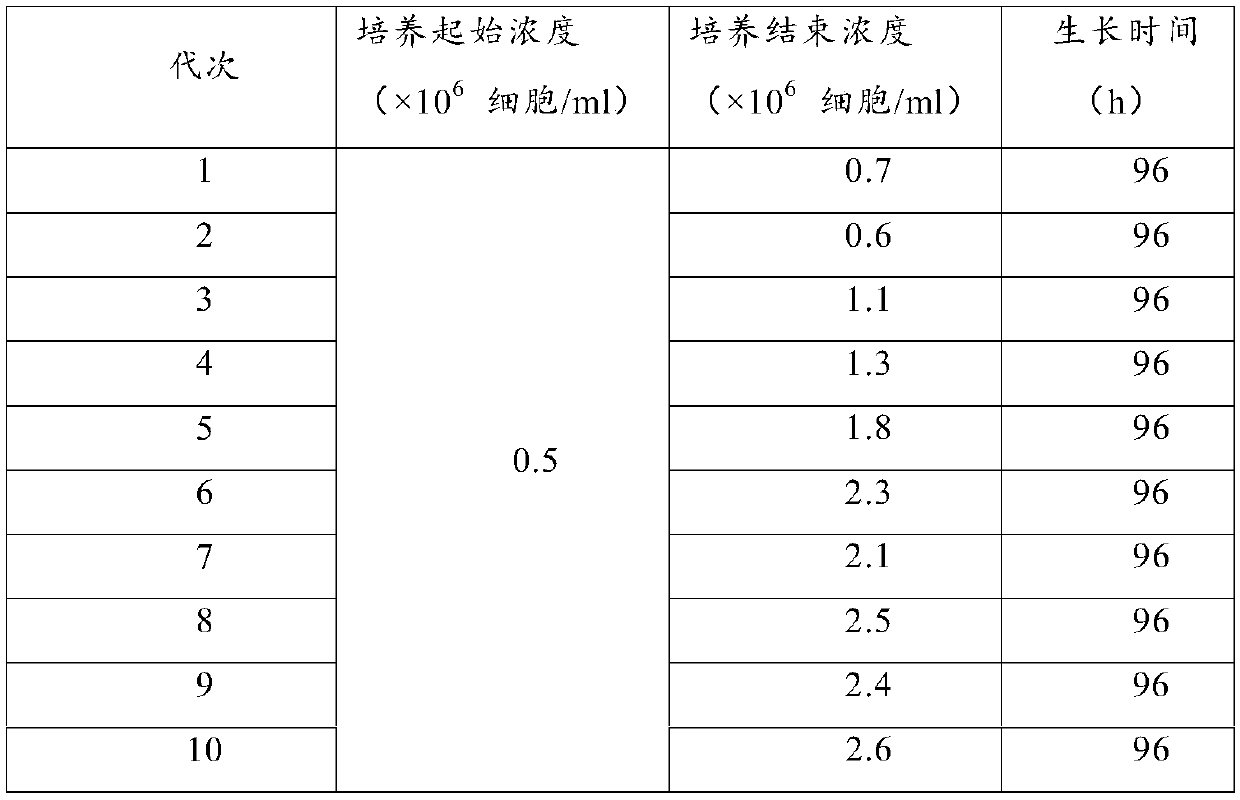
The invention discloses a sheep testis immortalized cell domestication method suitable for full suspension culture. The method comprises the following steps: (1) carrying out water bath resuscitationon undomesticated sheep testis cells, and centrifuging; (2) resuspending the cells with a DMEM culture solution containing 5% of newborn bovine serum, inoculating the cells into a cell culture bottle,and culturing; (3) subculturing, and digesting; (4) carrying out cyclotron suspension culture; and (5) transferring into a suspension culture bottle, culturing until the cell mass disappears, and ensuring that the cell density can be stable during repeated culture so as to obtain the sheep testis immortalized cells adapting to full suspension. Under the condition of suspension culture, the passage cells domesticated and cultured by the method are spherical and transparent, and are in a single or small-ball suspension state; the suspension domesticated cells subjected to 10 generations can bestably cultured to 2-3*10<6> cells / ml within 96 hours; and the virus culture adaptability results show that the virus titer reaches the peak value and is similar to the virus production level of adherent culture cells after the domesticated suspension cells are inoculated with orf virus for 72 hours.
Owner:安徽东方帝维生物制品股份有限公司
Orf virus-based platform for vaccine delivery
ActiveUS20200188509A1Enhance immune responseEfficient replicationSsRNA viruses negative-senseSsRNA viruses positive-senseHeterologousDisease
The present invention is directed to novel vaccine delivery platform based on the Orf virus (ORFV) genome, which carry heterologous antigens, methods of making and methods of using the same for prevention of infections, diseases, and other conditions in animals.
Owner:SOUTH DAKOTA BOARD OF REGENTS +1
Sheep pox and aphtha bigeminy cell attenuated vaccine and preparation method and application thereof
ActiveCN106139141AAvoid the risk of carrying other pathogensQuality improvementPowder deliveryViral antigen ingredientsAttenuated vaccineSheeppox
The invention discloses a sheep pox and aphtha bigeminy cell attenuated vaccine and a preparation method and application thereof. The effective ingredient of the sheep pox and aphtha bigeminy cell attenuated vaccine is sheep pox virus and contagious pustular dermatitis virus cell passage attenuate virus or passage virus thereof which are separated through the inventor and obtained after passage attenuate culture, wherein the sheep pox virus cell passage attenuate virus is Sheeppox Virus GS-WW 2010 F44 strain, the preservation number of CCTCC NO: V201504, the sheep pox virus (contagious pustular dermatitis virus) cell passage attenuate virus is Orf Virus HB-TS09F65 strain, and the preservation number is CCTCC NO:V201406. Toxin attacking protection test results show that the sheep pox and aphtha bigeminy cell attenuated vaccine is safe and capable of producing effective immune protection force. Accordingly, the sheep pox and aphtha bigeminy cell attenuated vaccine is of an important practical significance in researching safe and efficient attenuated vaccine and preventing and controlling sheep pox and aphtha bigeminy.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Sheep aphthous virus virulence gene vir deletion mutant strain and preparation method and application thereof
InactiveCN104878043BLow toxicityImproving immunogenicityAntiviralsViruses/bacteriophagesVirulent characteristicsShuttle vector
The invention relates to a mutant strain of the virulence gene VIR of aphthous ulcer virus and its preparation method and application. The preparation method comprises the method of cloning the gene fragment containing the VIR virulence gene of ORFV-SHZ1 strain and the flanking sequence by using the PCR method, and deleting it by the enzymatic cutting method. Remove the VIR virulence gene, insert the expression cassette with the late promoter P11 of vaccinia virus and the LacZ reporter gene at the deletion site, and construct a recombinant shuttle vector with the LacZ gene as a selection marker; transfect the recombinant shuttle vector into ORFV-infected Vero Homologous recombination was carried out in the cells, and the recombinant virus ORFV-ΔVIR-LacZ with deletion of the VIR virulence gene was obtained by plaque screening and purification. The invention quickly reduces the virulence of ORFV by means of genetic engineering, provides technical support for the research and development of ORF vaccines, has the characteristics of short production cycle, no need for domestication, convenient and quick operation, etc., and can be widely used.
Owner:SHIHEZI UNIVERSITY
Orf F1L vaccine based on ferritin nanoparticles and preparation method of orf F1L vaccine
ActiveCN110478480AResolution cycleSolve the problem of weak immunogen response and low responseViral antigen ingredientsVirus peptidesGenetic engineeringOrf virus
The invention discloses an orf F1L vaccine based on ferritin nanoparticles and a preparation method of the orf F1L vaccine. According to the invention, a fusion protein is characterized in that an orfF1L protein is externally connected to the surface of ferritin through a genetic engineering method to form nanoparticles, namely the orf F1L / Fe fusion protein. The target protein provided by the invention has good immunogenicity and can be used for preventing infection of the orf virus.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Goat testis fibroblast membrane system yeast cDNA library and construction method thereof
ActiveCN108998443AOvercome limitationsLibrary creationProtein nucleotide librariesBiotechnologyCDNA library
The invention provides a preparation method for a goat testis fibroblast membrane system yeast cDNA library. The preparation method comprises the following steps: preparation of goat testis fibroblasts; extraction of RNAs of the goat testis fibroblasts; synthesis of cDNAS and homogenization of a cDNA library; linkage of the cDNAS with a pPR3-N vector, transformation of the host cell DH5alphja witha linkage product so as to obtain the goat testis fibroblast membrane system yeast cDNA library, and cloning and verification; screening of the goat testis fibroblast membrane system yeast cDNA library; etc. The goat testis fibroblast membrane system yeast cDNA library prepared in the invention has a cDNA storage capacity of 7.5*10<5>; fragments have sizes in a range of 200 to 2000 bp and an average length of 1000 bp; and the recombination rate of the library is 100%. The screening of the goat testis fibroblast membrane system yeast cDNA library with the membrane protein of Orf viruses provesthat the library is a high-quality library.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
LAMP visual detection kit for orf virus
PendingCN111996287AAvoid formingGuaranteed accuracyMicrobiological testing/measurementDNA/RNA fragmentationMolecular biologyOrf virus
The invention relates to a LAMP visual detection kit for an orf virus. A detection primer in the kit can specifically detect the orf virus aiming at a conservative VIR sequence of the orf virus; a detection means of constant-temperature reaction is adopted, so that the whole reaction can be realized only by heating, and the dependence of a traditional nucleic acid detection technology on a PCR instrument is eliminated; the LAMP detection kit can realize rapid on-site detection of the orf virus, and a detection result can be directly observed by visual inspection, so that the LAMP detection kitis suitable for on-site detection; and the method is high in detection sensitivity, high in specificity, good in repeatability and high in detection speed, and can be used as the effective basic detection means for the orf virus.
Owner:陕西诺威利华生物科技有限公司 +1
Goat testis subculture cells that can be subcultured and their subculture and domestication method, special culture system and application
ActiveCN107058212BExtended passage timesSuitable for mass productionMicroorganism based processesArtificial cell constructsTGE VACCINELesion
The invention discloses a passage culture type sheep testis cell and a passage domesticating culture method, and a special culture system and application thereof, and provides a passage culture type sheep testis cell LT-1 (CGMCC No.12988) and a method for obtaining the cell by domesticating culture. The method has the advantages that optimized cell culture liquid is used for performing passage domesticating culture on a sheep testis primary cell (LT), the passage times of the sheep testis primary cell can be increased, and the method is suitable for batched production; the sheep orf virus is subject to multiplication culture by the cell; proofed by results, the sheep orf virus can be continuously subject to passage culture for three generations, the obvious lesion is produced, the content of virus reaches 107.5TCID50 / mL, and the method can be applied to the multiplication culture and vaccine preparation of the sheep orf virus.
Owner:JINYUBAOLING BIO PHARMA CO LTD
A dual-cell attenuated vaccine for sheep pox and aphthous ulcers and its preparation method and application
ActiveCN106139141BGuaranteed immune protectionSave solutionPowder deliveryViral antigen ingredientsSheeppoxTGE VACCINE
The invention discloses a sheep pox and aphtha bigeminy cell attenuated vaccine and a preparation method and application thereof. The effective ingredient of the sheep pox and aphtha bigeminy cell attenuated vaccine is sheep pox virus and contagious pustular dermatitis virus cell passage attenuate virus or passage virus thereof which are separated through the inventor and obtained after passage attenuate culture, wherein the sheep pox virus cell passage attenuate virus is Sheeppox Virus GS-WW 2010 F44 strain, the preservation number of CCTCC NO: V201504, the sheep pox virus (contagious pustular dermatitis virus) cell passage attenuate virus is Orf Virus HB-TS09F65 strain, and the preservation number is CCTCC NO:V201406. Toxin attacking protection test results show that the sheep pox and aphtha bigeminy cell attenuated vaccine is safe and capable of producing effective immune protection force. Accordingly, the sheep pox and aphtha bigeminy cell attenuated vaccine is of an important practical significance in researching safe and efficient attenuated vaccine and preventing and controlling sheep pox and aphtha bigeminy.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
A kind of goat pox, sheep pox bivalent cellular attenuated vaccine and its preparation method and application
ActiveCN104800842BGuaranteed immune protectionSave solutionAntiviralsAntibody medical ingredientsSheeppoxAttenuated vaccine
The invention provides a goatpox and sheep pox bivalent cell attenuated vaccine. The goatpox and sheep pox bivalent cell attenuated vaccine comprises effective ingredients, namely, a goatpox virus cell passage attenuation virus and a sheep pox virus cell passage attenuation virus, wherein the goatpox virus cell passage attenuation virus is a goatpox virus cell passage attenuation vaccine strain Goatpox Virus HN-XY2010F43 strain CCTCC V201503, and the sheep pox virus cell passage attenuation virus is a sheeppox virus cell passage attenuation vaccine strain Sheeppox Virus GS-WW2010F44 strain CCTCC V201504. The goatpox and orf virus bivalent cell attenuated vaccine is safe, can have the effective immune protection force and has the important practical significance in development of safe and efficient attenuated vaccines as well as prevention and control on goatpox and sheeppox through large-scale production of goatpox and sheep pox virus bivalent cell attenuated vaccines by the aid of the attenuation virus strains.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Kit for detecting orf virus of sheep and detection method thereof
ActiveCN103255234BStrong specificityHigh sensitivityMicrobiological testing/measurementFluorescence/phosphorescenceA-DNAVirology
The invention provides a detection method for detecting the orf virus of sheep. Real-time fluorescent quantitative PCR (polymerase chain reaction) detection is performed on a DNA (deoxyribonucleic acid) template of a sample by designing a pair of primers and a probe. The invention further provides a kit for detecting the orf virus of the sheep by utilizing the detection method. The real-time quantitative method which is established against H2L genes in a conserved region of the ORFV (orf virus) is high in specificity and good in stability. The kit for detecting the orf virus of the sheep, provided by the invention, is simple and convenient to operate, a user only needs to add DNA of the sample to be detected into a reaction tube, detection results can be obtained according to an amplification curve, the operation is time-saving and labor-saving, the cost is lower, and the time is shortened from original 3-4 hours to 1-2 hours; and furthermore, the sensitivity is higher than that of the ordinary PCR, and the detection method and the kit can be used for detecting the low content of the orf virus of the sheep, can be suitable for all levels of prevention and control units, basic-level veterinary stations, large and medium-sized breeding farms and the like in China, and has better application prospects.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
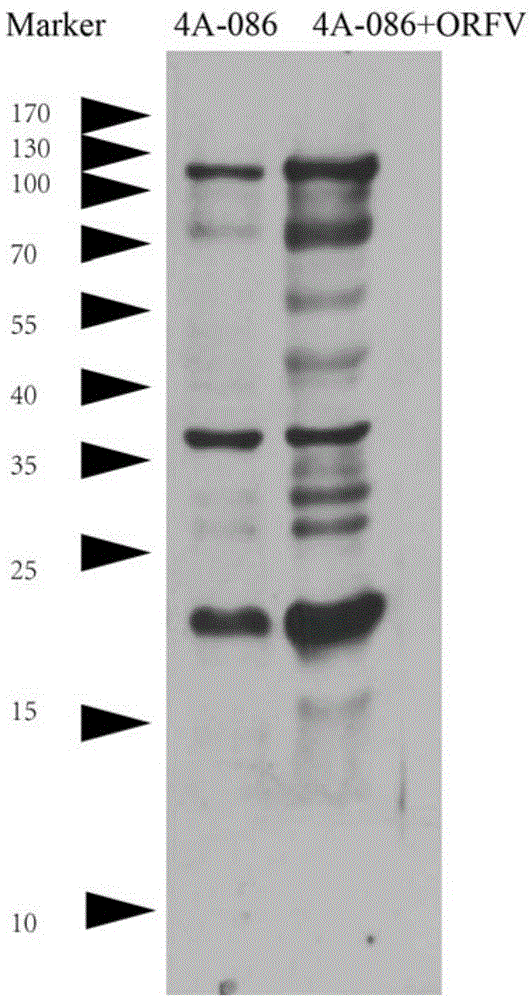
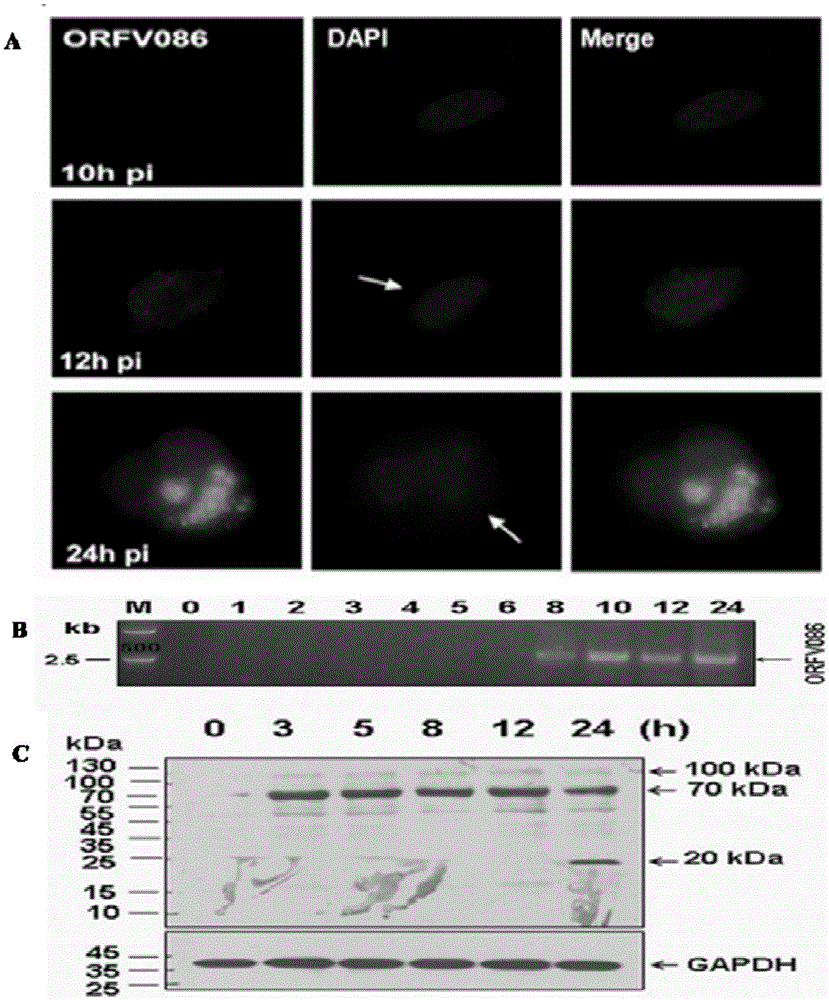
A kind of monoclonal antibody hybridoma cell line 2g8d10 of orfv086 protein of sheep oral ulcer virus and its monoclonal antibody
ActiveCN104046594BImprove featuresHigh sensitivityImmunoglobulins against virusesMicroorganism based processesImmunofluorescenceWestern blot
The invention discloses an orf virus protein ORFV086 monoclonal antibody hybridoma cell strain 2G8D10 and a monoclonal antibody thereof. A collection number of the hybridoma cell strain 2G8D10 is CCTCC NO: C201471. The monoclonal antibody is secreted from the hybridoma cell strain 2G8D10 with the collection number of CCTCC NO: C201471. The monoclonal antibody can carry out high-sensitivity specific antigen-antibody reaction with the orf virus protein ORFV086, and can be effectively used for carrying out biological diagnosis including ELISA (enzyme-linked immuno sorbent assay), western-blot, immunofluorescence and immunohistochemical detection onto the orf virus protein ORFV086.
Owner:GUANGZHOU HONGXIANG BIOMEDICAL TECH
A kind of goat pox, sheep pox, aphthous triple cell attenuated vaccine and its preparation method and application
ActiveCN106075429BGuaranteed immune protectionSave solutionViral antigen ingredientsAntiviralsSheeppoxAttenuated vaccine
The invention discloses a goatpox, sheeppox and contagious ecthyma triple cell attenuated vaccine and a preparation method and application thereof. The effective components of the vaccine are separated by an inventor automatically, and after attenuation culture is conducted, goatpox virus, sheeppox virus and Orf virus cell passage attenuated viruses or passage viruses thereof are obtained, wherein a goatpox virus is a Goatpox Virus HN-XY2010F43 strain, a sheeppox virus is a Sheeppox Virus GS-WW 2010 F44 strain, and an Orf virus is an Orf Virus HB-TS09F65 strain. It is shown through attacking protection test results that the prepared goatpox, sheeppox and contagious ecthyma triple cell attenuated vaccine is safe and capable of producing an effective immune protection force, and important practical significance in developing safe and efficient goatpox, sheeppox and contagious ecthyma triple cell attenuated vaccine and preventing and controlling goatpoxes and ecthyma is achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Orf virus attenuated strain and application thereof
InactiveCN111139226AHigh-quality material basePremium Choice SpaceViral antigen ingredientsInactivation/attenuationTGE VACCINEAphthovirus
The invention provides an orf virus attenuated strain and an application thereof. The attenuated strain is an orf virus attenuated strain QFnm2015, the preservation institution is the China Center forType Culture Collection, and the preservation number is CCTCC NO: V201903. According to the invention, the isolated orf virus is proliferated and cultured on lamb testicular cells, and the orf virusattenuated strain is cultured through continuous passage. The isolated strain of the orf virus provides a high-quality material basis and selection space for developing an effective vaccine of endemicORFV, and has important significance for prevention and treatment of orf.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
A Virus Isolation Method for Low-Content Samples of Aphthus Virus
ActiveCN104745539BExpand the scope of materialsShort separation cycleMicroorganism based processesViruses/bacteriophagesFreeze thawingPrimary cell
The invention relates to a virus isolation method of a low-content sample of aphthus virus. Traditional aphthus virus is isolated from scabs and lip dander of diseased sheep. The separation cycle is long, and it takes 5 generations of blind transmission before lesions appear. research work. The present invention uses a unique Virus holder solution to centrifugally filter samples such as saliva, milk, and viscera, inoculate them in the primary cells of the bovine testis and discard the venom after culturing; add the cell maintenance solution to collect the poison after culturing; freeze and thaw repeatedly to collect the cells In the culture, cytopathic changes can be observed 72 hours after inoculation with bovine testis cells in the third passage. The invention uses a unique Virus holder solution to process samples, can isolate aphthus virus from samples with extremely low virus content, has a wide range of materials, short virus isolation period, high stability, and can well maintain the integrity and activity of the virus.
Owner:NORTHWEST A & F UNIV
Recombinant DNA vaccine for resisting ecthyma and sheep pox and recombinant plasmid thereof
The invention provides a recombinant DNA vaccine and a recombinant plasmid thereof, the DNA vaccine recombinant plasmid comprises an antigen coding sequence and a plasmid vector, the antigen coding sequence comprises an orf antigen coding sequence and a sheep pox antigen coding sequence which are connected in series, and an orf antigen and a sheep pox antigen are expressed as independent antigen proteins. The invention provides construction of a double-gene recombinant DNA (Deoxyribose Nucleic Acid) vaccine of an orf virus gene and a sheep pox virus gene and analysis of a mouse immune effect. According to the invention, an orf virus B2L (011) gene and a sheep pox virus P32 gene are selected, the two genes are connected in series by using a self-cleaving peptide P2A, and the self-cleaving peptide P2A generates self-cleaving in eukaryotic cells, so that two antigen proteins are independently expressed on an eukaryotic expression vector pcDNA3.1 (+). The recombinant DNA vaccine prepared by the invention also has a better immune effect after being used for immunizing a mouse, and can be used as a reference of a candidate vaccine.
Owner:JILIN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com