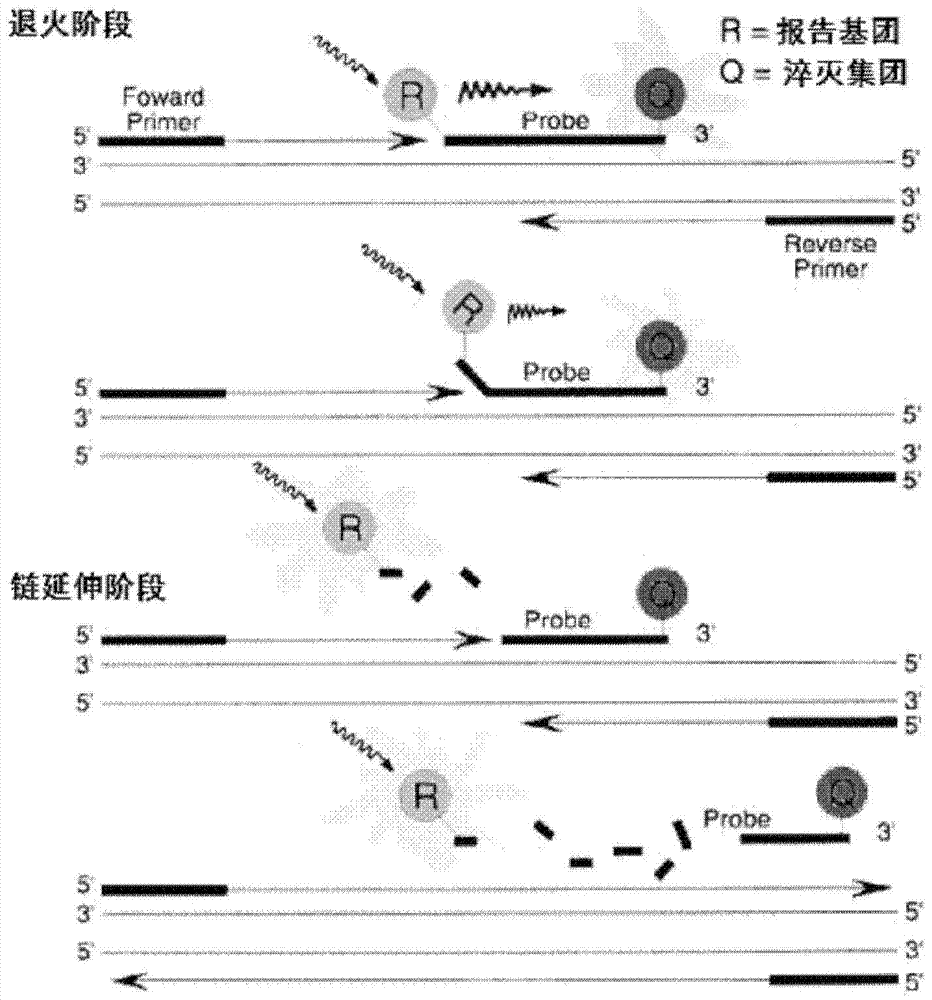
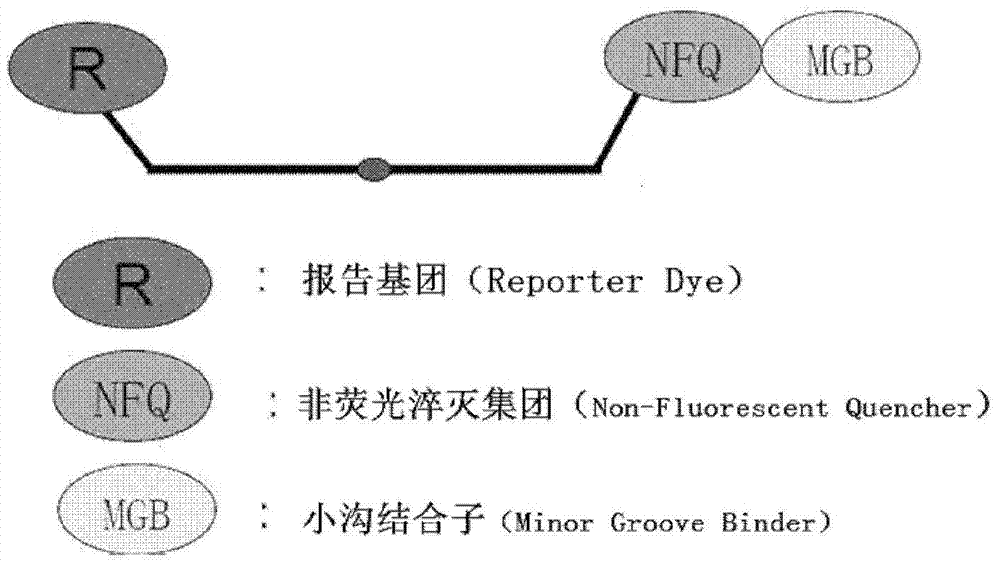
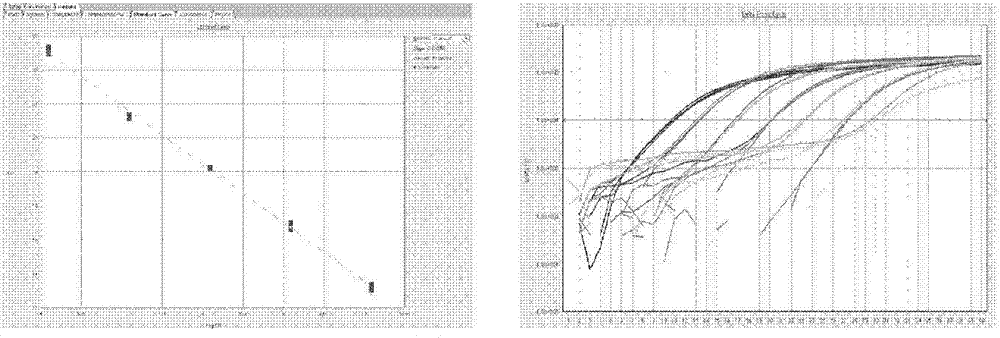
Bovine viral diarrhea virus (BVDV) fluorescent quantitative RT-PCR (reverse transcription-polymerase chain reaction) detection kit
A technology for bovine viral diarrhea and a detection kit, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, etc., and can solve problems such as laborious, time-consuming, and low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] ——Clinical testing
[0110] The BVDV-MGB-RT-PCR detection kit was used to detect 94 finished and semi-finished samples of swine fever vaccines from 8 manufacturers of live swine fever vaccines. At the same time, the RT-PCR method (see note) was used as the conformity to evaluate the method. Feasibility of clinical promotion.
[0111] (1) Extraction of RNA
[0112] RNA was extracted using commercial kits or conventional RNA extraction methods. The extracted RNA must be amplified within 2 hours.
[0113] (2) Amplification reagent preparation
[0114] Take out each component from the kit, melt at room temperature, and centrifuge at 2000r / min for 5s. The preparation of each sample test reaction system is shown in Table 3.
[0115] (3) Nucleic acid amplification and detection
[0116] Put the above-mentioned PCR tubes into the fluorescent PCR detector, and record the order in which the samples are placed.
[0117] Loop condition settings:
[0118] In the first stage,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com