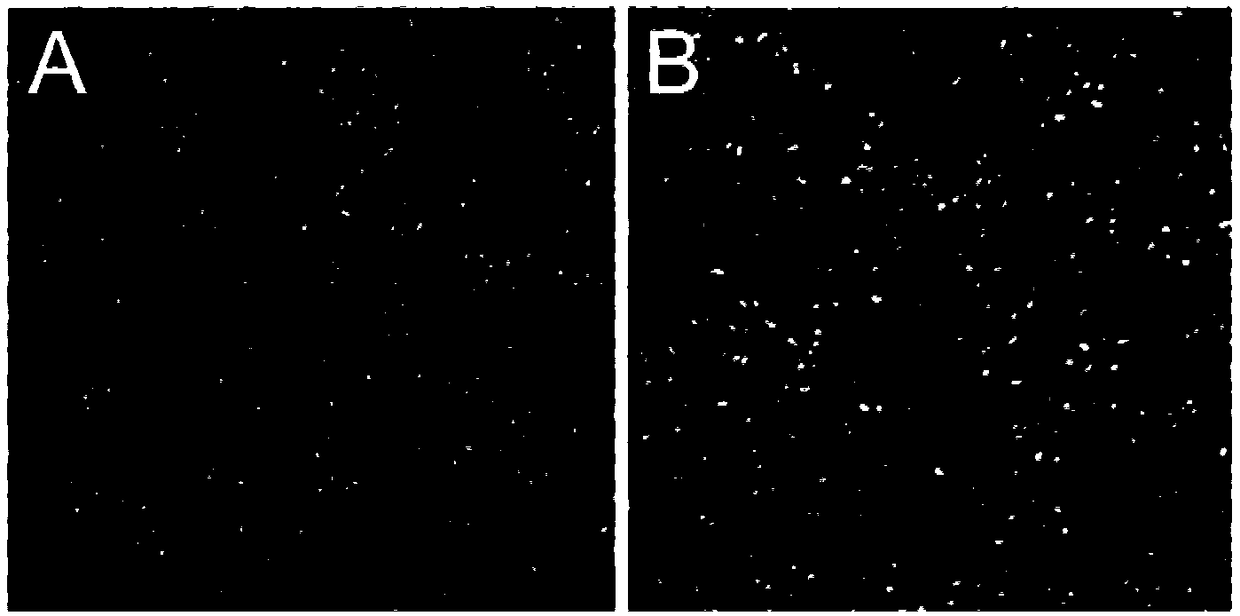
Bovine viral diarrhea virus-like particle and construction method and application thereof
A technology of bovine viral diarrhea and its construction method, which is applied in the field of bovine viral diarrhea virus-like particles and its construction, can solve the problems that the research on BVDV virus-like particles has not yet been carried out, and achieve the effect of low cost and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The construction of embodiment 1 recombinant transfer vector
[0051] 1. Acquisition of BVDV structural gene
[0052] There are many BVDV strains, but the structure and function are basically the same. In the present invention, the original nucleotide sequence of the C-E0-E1-E2-P7 gene region of the strain with GenBank accession number U63479.1 is selected as the research object and optimized. Synthesized, the nucleotide sequence was synthesized by Nanjing GenScript with a length of 2910bp, and the optimized and synthesized target sequence was connected to the PUC57 vector (purchased from Thermo Fisher Scientific), named PUC57-C-E0-E1-E2- P7.
[0053] The original nucleotide sequence of the BVDV C-E0-E1-E2-P7 gene region is shown in SEQ ID NO:1, and the amino acid sequence encoded by them is shown in SEQ ID NO:2. The optimized and synthesized nucleotide sequence is shown in SEQ ID NO:3, and the amino acid sequence encoded by them is shown in SEQ ID NO:4.
[0054] 2. ...
Embodiment 2
[0060] The construction of embodiment 2 recombinant baculovirus
[0061] 1. Construction of recombinant baculovirus genome
[0062] In accordance with the operating instructions of Invitrogen, briefly described below. The pFastBac1-C-E0-E1-E2-P7 positive plasmid obtained in Example 1 was transformed into Escherichia coli DH10Bac competent cells (containing the Autographa californica nuclear polyhedrosis baculovirus AcMNPV genome, Invitrogen Cat NO.10359-016 ), the conversion conditions are as follows:
[0063] Mix 2 μL of pFastBac1-C-E0-E1-E2-P7 positive plasmid with 100 μL of Escherichia coli DH10Bac competent cells, and then bathe in ice for 35 minutes. Resistant LB medium, after culturing at 37°C and 200rpm for 4-5 hours, the bacterial solution was subjected to 10 -1 、10 -2 、10 -3 Doubling serial dilutions were taken, and 100 μL of each dilution was spread on a culture dish containing three-antibody-resistant bacteria (containing 50 μg / mL kanamycin, 7 μg / mL gentamicin,...
Embodiment 3
[0066] Embodiment 3 Rescue of recombinant baculovirus
[0067] In accordance with the Invitrogen company's expression system and liposome Lipfectin 2000 operating instructions, briefly described as follows. The recombinant baculovirus Bacmid plasmid that embodiment 2 extracts is transfected insect cell Spodoptera frugiperda (Spodopterafrugiperda) Sf9 cell line, and transfection process is as follows:
[0068] (1) Add 4 μg of the recombinant baculovirus Bacmid plasmid to 250 μL of Grace medium without serum and double antibody, mix well, and let stand at room temperature for 5 minutes;
[0069] (2) Add 6 μL liposome Lipfectin 2000 to 250 μL Grace culture medium without serum and double antibody, mix well, and let stand at room temperature for 5 minutes;
[0070] (3) Mix equal volumes of the above (1) and (2) solutions, and let stand at room temperature for 20 minutes;
[0071] (4) Sf9 cells cultured in a 6-well plate (covering 70-80% of the bottom area of the well, purchase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com