Methods and compositions for immunizing pigs against porcine circovirus
A technology of porcine circovirus and composition, applied in the direction of antiviral agent, virus, drug combination, etc., can solve the problem of specific protein paradigm without immunogenic properties and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
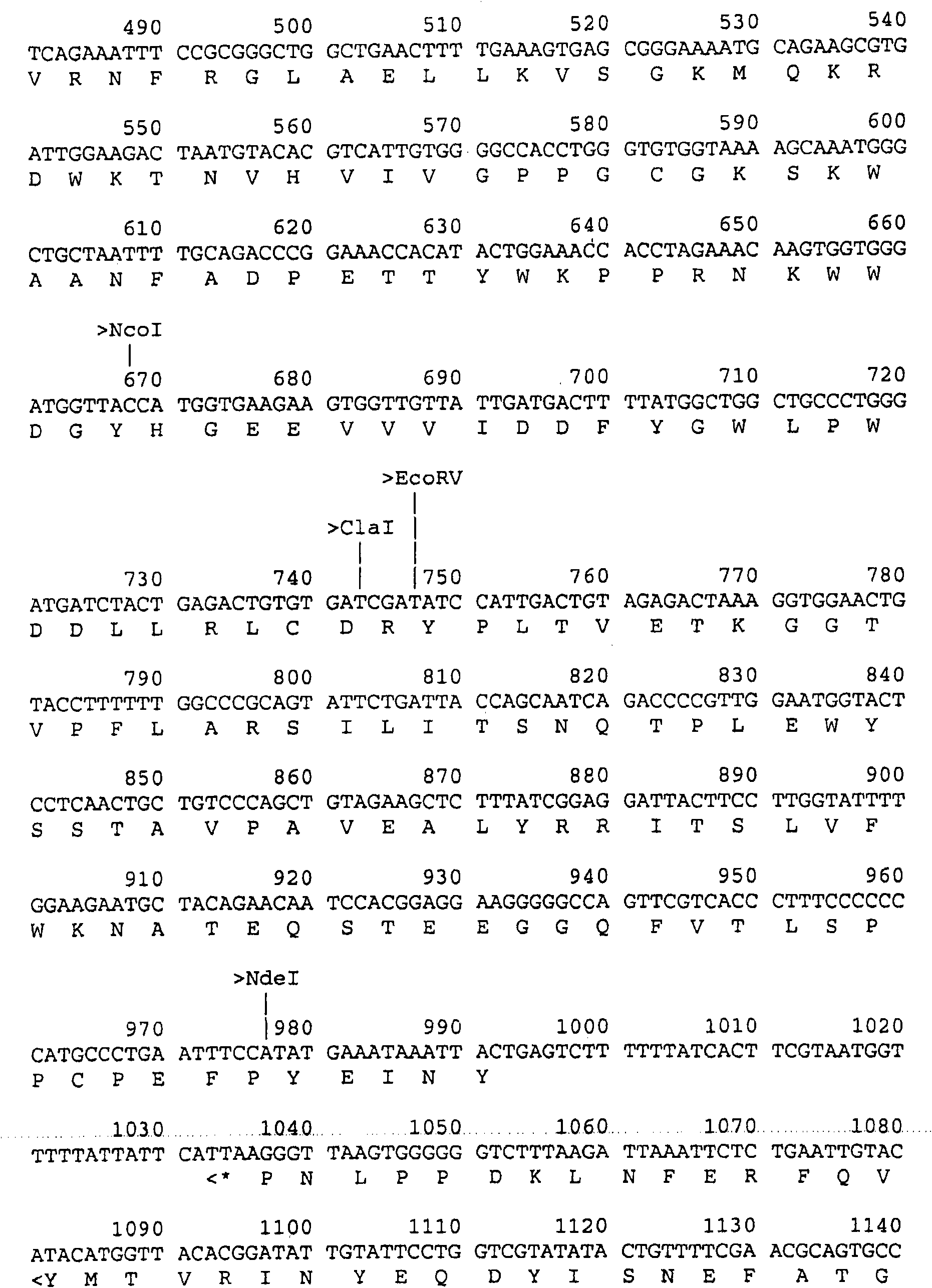
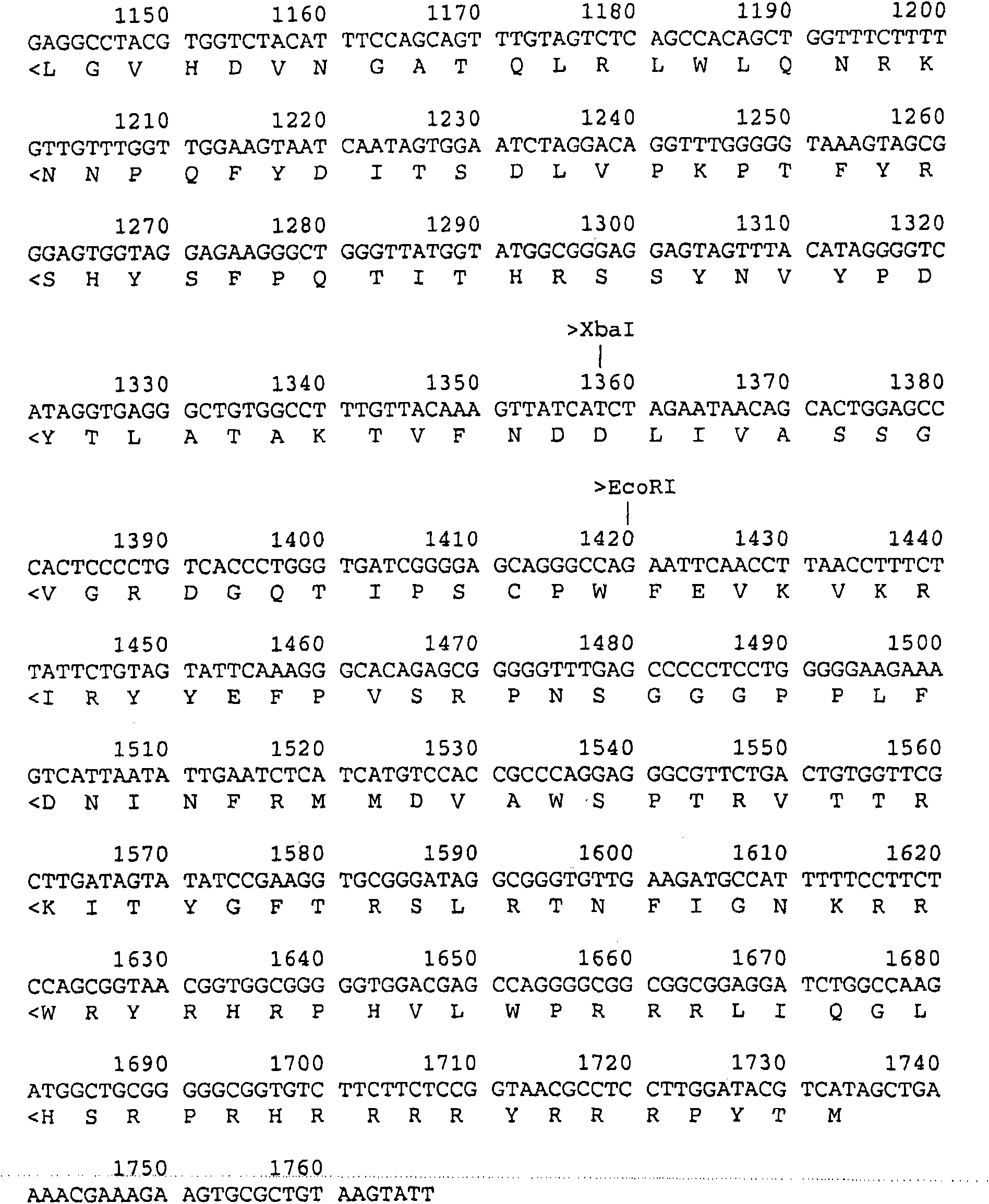
[0131] Subunit vaccines are generally prepared differently than modified live or inactivated vaccines. Before subunit vaccines can be prepared, the protective or antigenic components of the vaccine must be identified. These protective or antigenic components include specific amino acid segments or fragments of viral capsid proteins, which elicit particularly strong protective or immunogenic responses in pigs; single or multiple viral capsid proteins themselves, which oligomeric bodies, and higher-level associations of viral capsid proteins that form viral substructures or identifiable parts or units of these substructures; oligoglycosides, glycolipids present on or near the viral surface or within viral substructures Or glycoproteins, such as lipoproteins or lipid groups associated with viruses, and so on. Preferably, a capsid protein, such as the protein encoded by the ORF2 gene, is used as an antigenic component of a subunit vaccine. Other proteins encoded by infectious DN...
Embodiment 1
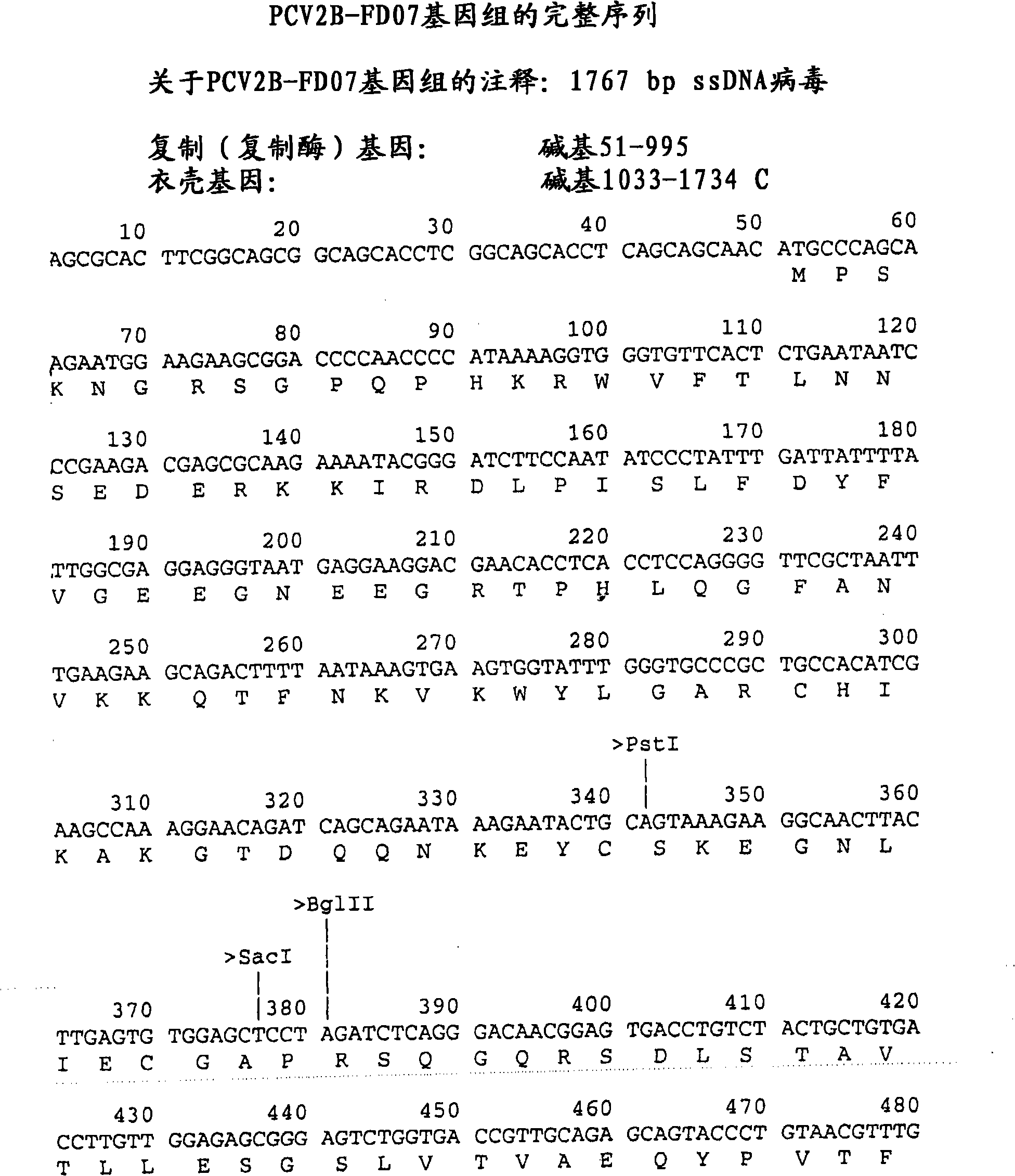
[0161] Isolation and identification of two novel porcine circovirus type 2B strains
[0162] A study was planned to test a novel vaccine formulation in pigs to assess its efficacy against porcine circovirus and Chlamydia hyopneumoniae. During the course of the study, several pigs in the control and vaccinated groups were observed to show symptoms of PMWS. Environmental exposure of these pigs to PCV2 prior to challenge was then confirmed. Molecular analysis of blood and tissue samples from these pigs revealed that they had a type 2B strain that was different from the strain used for the challenge. Furthermore, sequence analysis established that the PCV2B strain annotated FD07 and another novel PCV2B pig (annotated FDJE) isolated from pigs on the farm were distinct from other type 2B strains identified in other field studies and previously identified by others. Materials and methods used to isolate and characterize these two novel PCV2B strains are described below.
[0163] M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mean titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com