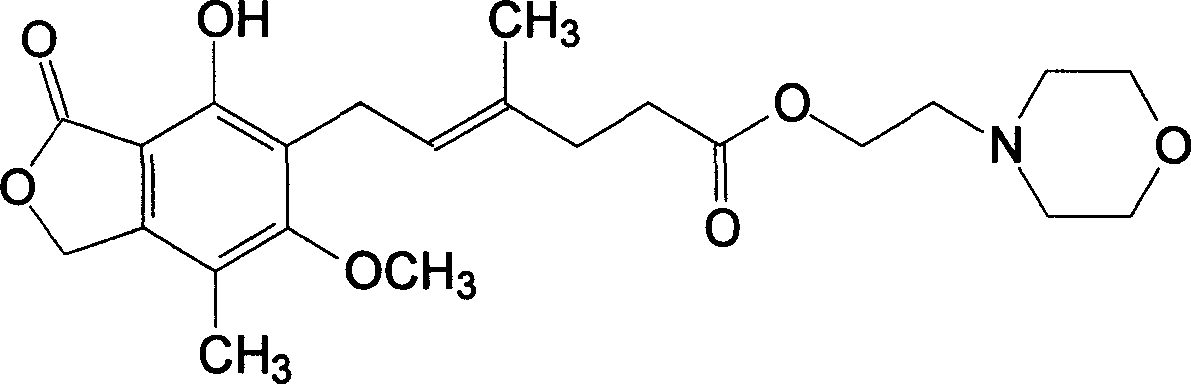
Prepn process of Mycophenolate mofetil
A technology of mycophenolate morphinate and mycophenolic acid, which is applied in the field of preparation of mycophenolate morphinate, can solve the problems of long time and high reaction temperature, achieve the effect of low reaction temperature and solve the problem of product color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
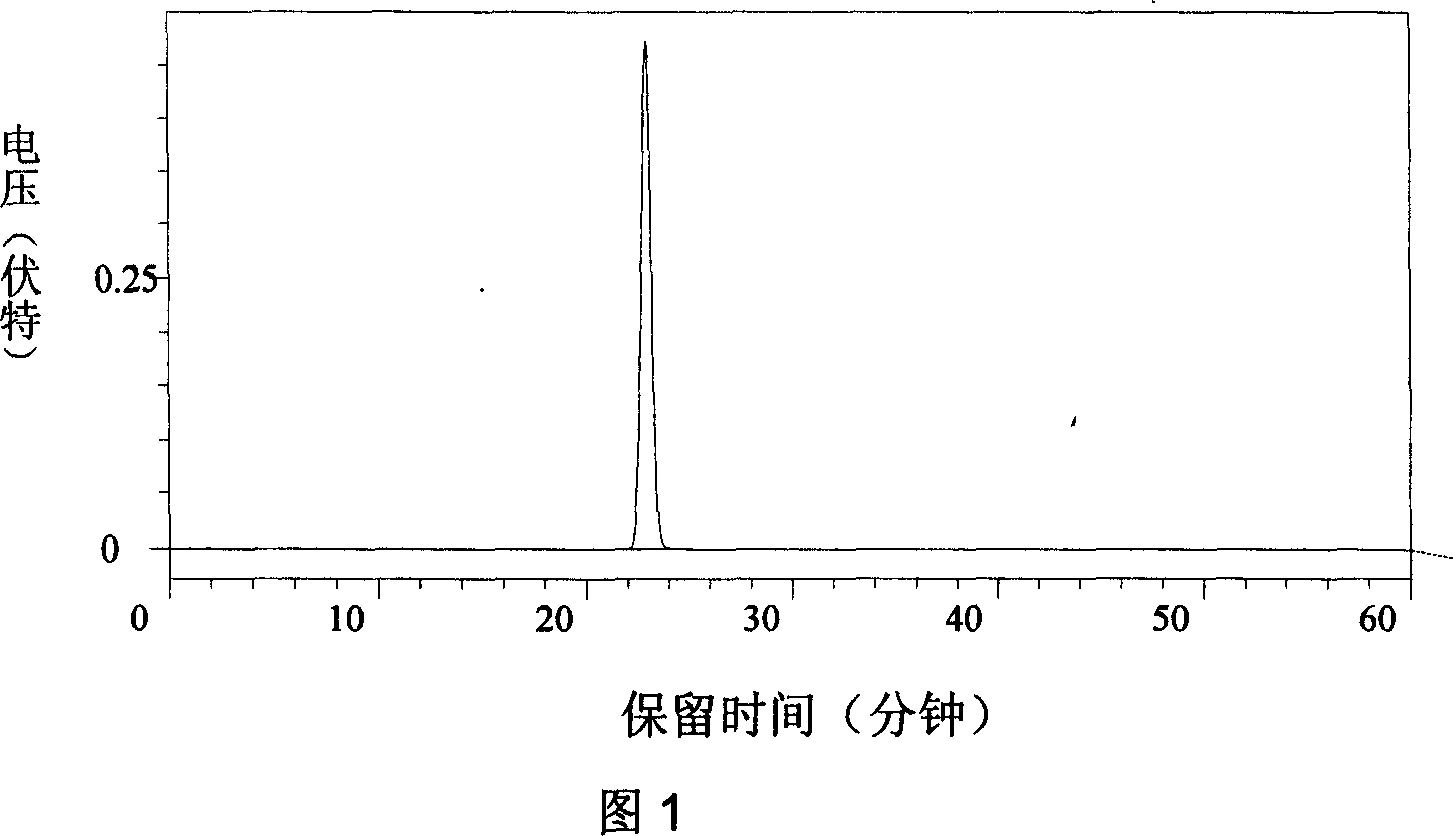
[0022] Add 30ml of tetrahydrofuran and 10g of mycophenolic acid to the reactor with reflux cooler, heat to about 50℃, mechanically stir until completely dissolved, then add 11.5ml of morpholine ethanol to the reactor, and then put 0.8 in the reactor g catalyst zinc oxide; heated to 67°C, refluxed for 24 hours, filtered to remove the catalyst zinc oxide; evaporated the organic solvent under reduced pressure to obtain crude mycophenolate mofetil, add 250ml ethyl acetate to dissolve the crude product, and then use 250ml K with a mass percentage concentration of 5% 2 CO 3 The solution is washed once, and then washed with 250ml purified water; it is concentrated to a mass percentage of 30% at a temperature of 40℃, cooled to 10℃, crystallized for 15 hours, and crystals are precipitated; filtered, and then washed with 10ml of cold ethyl acetate , Vacuum drying at a temperature of 40°C, and finally 11.5 g of a refined product with a purity of ≥99.7% (HPLC), with a yield of 85%. see pictur...
Embodiment 2
[0024] A preparation method of mycophenolate mofetil, including the following steps:
[0025] (1) Dissolve mycophenolic acid in tetrahydrofuran at a ratio of 1g:2ml in a reactor with a reflux cooler, heat to about 50°C, and stir until dissolved;
[0026] (2) Add morpholine ethanol at a ratio of 1:3 in molar ratio of mycophenolic acid to morpholine ethanol, and then add magnesium oxide as a catalyst, so that the added mass of the catalyst is 5% of the mass of mycophenolic acid;
[0027] (3) Raise the temperature to 67°C and reflux for 24 hours;
[0028] (4) Filter, remove the catalyst, and evaporate the organic solvent under reduced pressure to obtain the crude mycophenolate mofetil.
[0029] Add ethyl acetate to dissolve the crude product according to the mass ratio of crude mycophenolate morphenate to ethyl acetate of 1:15; use 1.5 times the volume of the dissolving solution with a mass percentage of K with a concentration of 5% 2 CO 3 The solution is washed once with the dissolv...
Embodiment 3
[0031] A preparation method of mycophenolate mofetil, including the following steps:
[0032] (1) Dissolve mycophenolic acid in tetrahydrofuran at a ratio of 1g:8ml in a reactor with a reflux cooler, heat to about 50°C, and stir to dissolve;
[0033] (2) Add morpholine ethanol at a ratio of 1:2 in molar ratio of mycophenolic acid to morpholine ethanol, and then add zinc oxide as a catalyst, so that the added mass of the catalyst is 8% of the mass of mycophenolic acid;
[0034] (3) Raise the temperature to 70°C and reflux for 20 hours;
[0035] (4) Filter, remove the catalyst, and evaporate the organic solvent under reduced pressure to obtain the crude mycophenolate mofetil.
[0036] It can also include the following steps:
[0037] Add ethyl acetate to dissolve the crude product according to the mass ratio of crude mycophenolate morphenate to ethyl acetate of 1:25; use K with a mass percentage concentration of 5%, which is equivalent to 2 times the volume of the dissolving solutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com