Method for preparing mycophenolate mofetil
A technology of mycophenolate mofetil and ethyl acetate, which is applied in the field of preparing mycophenolate mofetil, can solve problems such as equipment corrosion, pollution, and difficult industrialization, and achieve the effects of low production cost and low process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
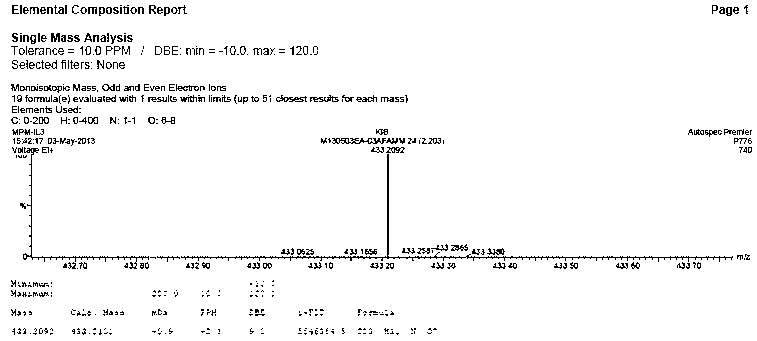
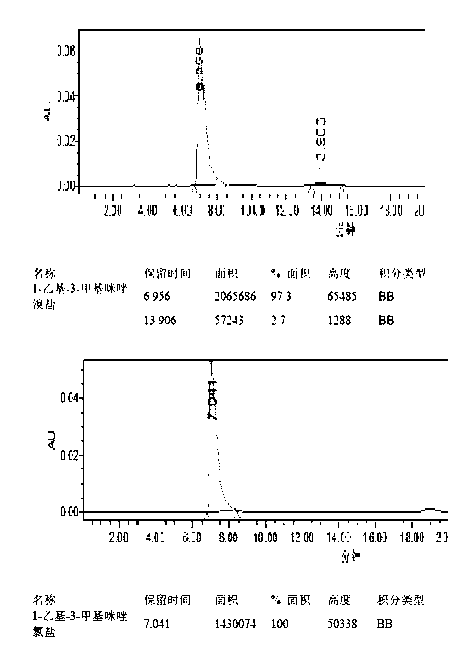
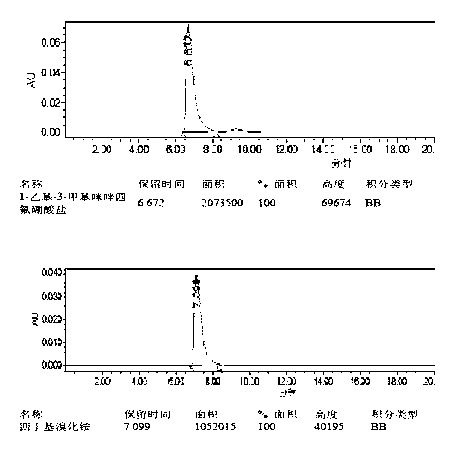
[0049] Under the protection of nitrogen, mix 20 g of mycophenolic acid and 40 mL of dibutyl ether, stir vigorously and heat up to 60°C, add 10 mL of 2-hydroxyethyl morpholine, react for 15 h under the condition of azeotropic water removal, and cool to room temperature and added 100 mL of dichloromethane. The solution was washed twice with 20mL dilute ammonia water (1% by mass concentration), washed twice with 50mL tap water, and the organic solvent was evaporated under reduced pressure. The residue was dissolved in a mixed solvent of propanol / ethyl acetate (150 mL:30 mL) (propanol / ethyl acetate=5:1) under nitrogen protection, and the temperature was raised to 50-60°C. Subsequently, 1-ethyl-3-methylimidazolium chloride (9mg / 1mmol / 2mL hot distilled water) was added, and when the purple color of the residue disappeared, it was filtered while hot, and the solid was washed with 33 mL propanol / ethyl acetate mixture ( 50-60°C) to wash and combine the filtrates.
[0050] (1) Slowly ...
Embodiment 2
[0053] Under the protection of nitrogen, mix 20 g of mycophenolic acid and 40 mL of dibutyl ether, stir vigorously and raise the temperature to 50-60 ° C, add 10 mL of 2-hydroxyethyl morpholine, and react for 15 hours under the condition of azeotropic water removal, The organic solvent was distilled off under reduced pressure, and 150 mL of ethyl acetate was added to the residual solid. The solution was washed twice with 20 mL of saturated sodium bicarbonate and twice with 50 mL of tap water, and the organic solvent was evaporated under reduced pressure. Under the protection of nitrogen, the residue was dissolved in a mixed solvent of propanol / ethyl acetate (150 mL:30 mL) (propanol / ethyl acetate=5:1, the temperature was raised to 50-60°C). Subsequently, 1-ethyl-3-methylimidazolium bromide (12mg / 1mmol / 2 mL hot distilled water) was added, and when the purple color of the residue disappeared, it was filtered while hot, and the solid was washed with 33 mL propanol / ethyl acetate mi...
Embodiment 3
[0055] Under the protection of nitrogen, mix 20 g of mycophenolic acid and 40 mL of dibutyl ether, stir vigorously and raise the temperature to 50-60 ° C, add 10 mL of 2-hydroxyethyl morpholine, under the condition of azeotropic water removal, reflux for 15 hours, Cool to room temperature and add 150 mL of ethyl acetate. The solution was washed twice with 20 mL of dilute ammonia water (1% by mass) and twice with 40 mL of tap water. The organic solvent was evaporated under reduced pressure. Under the protection of nitrogen, the residue was dissolved in a mixed solvent of propanol / ethyl acetate (150 mL:30 mL) (propanol / ethyl acetate=5:1, the temperature was raised to 50-60°C). Subsequently, tetrabutylammonium bromide (20 mg / 1mmol / 2 mL of hot distilled water) was added, and when the purple color of the residue disappeared, it was filtered while hot, and the solid was washed with 33 mL of propanol / ethyl acetate mixture (50-60°C ), combined the filtrates, added 0.1 g of pure myco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com