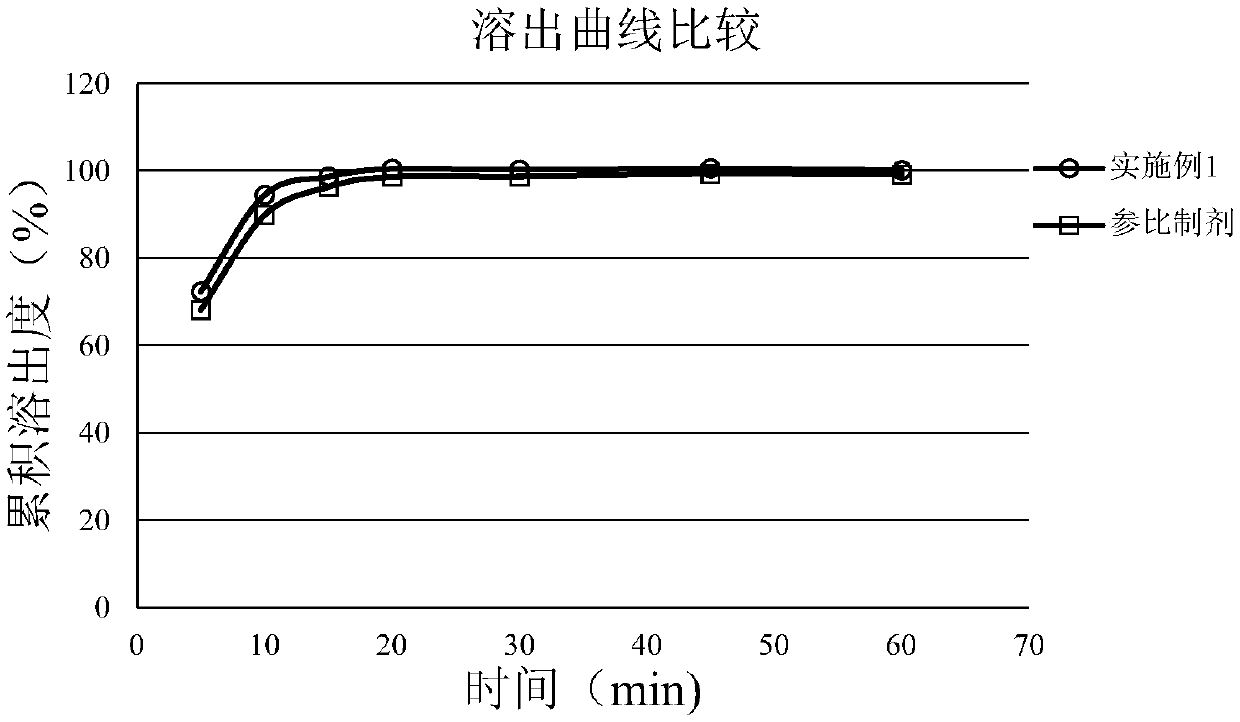
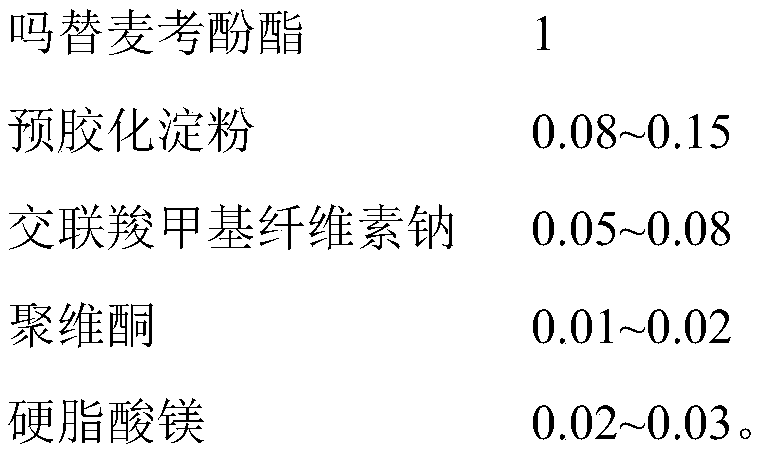
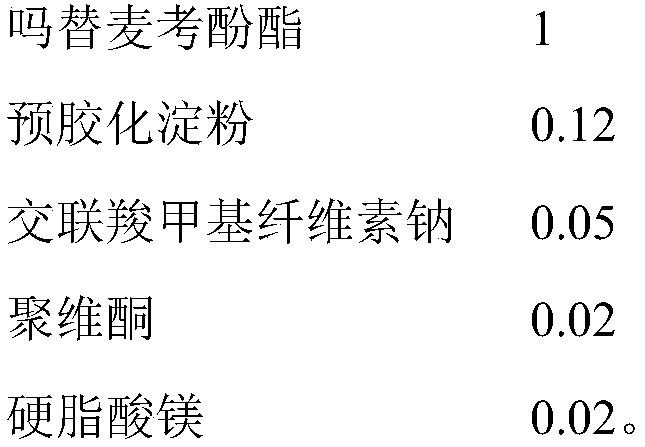
Mycophenolate mofetil capsule and preparation method thereof
A technology for mycophenolate mofetil and capsules, which is applied in the field of mycophenolate mofetil and its preparation, can solve problems such as bad smell, and achieve the effects of good fluidity, rapid dissolution and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Formula: mycophenolate mofetil 750g, pregelatinized starch 89.28g, croscarmellose sodium 35.7g, povidone K-90 17.85g, magnesium stearate 13.5g
[0036] Preparation Process:
[0037] a. According to the dosage of 3000 capsules, take 750g of mycophenolate mofetil, 89.28g of pregelatinized starch, 35.7g of croscarmellose sodium, 17.85g of povidone K-90, 13.5g of magnesium stearate g, pass through a 40 mesh sieve respectively for subsequent use;
[0038] b. Mix croscarmellose sodium and povidone K-90 for 3 minutes, then add pregelatinized starch and mix for 3 minutes, then add one-fifth of the raw materials and mix for 5 minutes; then add the remaining prescription amount of raw materials Mix for 10 min.
[0039] c. Add the above mixture into a wet granulator, start stirring (200rpm), add purified water with a total weight of 45% of raw and auxiliary materials, cut and make soft materials, and the obtained granules are granulated through a conical granulator;
[0040] d....
Embodiment 2
[0043] Formula: mycophenolate mofetil 750g, pregelatinized starch 60g, croscarmellose sodium 45g, povidone K-907.5g, magnesium stearate 18.75g
[0044] Preparation Process:
[0045] a. According to the dose of preparing 3000 capsules, take mycophenolate mofetil, pregelatinized starch, croscarmellose sodium, povidone K-90, and magnesium stearate, and pass through a 40-mesh sieve for use;
[0046] b. Mix croscarmellose sodium and povidone K-90 for 2 minutes, then add pregelatinized starch and mix for 2 minutes, then add one-fifth of the raw materials and mix for 3 minutes; then add the remaining prescription amount of raw materials Mix for 8 min.
[0047] c. Add the above mixture into the wet granulator, start stirring (300rpm), add purified water with 40% of the total weight of raw and auxiliary materials, cut the soft material, and the obtained granules are granulated through the cone granulator;
[0048] d. Fluidized bed drying (50°C, air intake frequency 40%) until the moi...
Embodiment 3
[0051] Formula: mycophenolate mofetil 750g, pregelatinized starch 112.5g, croscarmellose sodium 60g, povidone K-90 11.25g, magnesium stearate 22.5g
[0052] Preparation Process:
[0053] a. According to the dose of preparing 3000 capsules, take mycophenolate mofetil, pregelatinized starch, croscarmellose sodium, povidone K-90, and magnesium stearate, and pass through a 40-mesh sieve for use;
[0054] b. Mix croscarmellose sodium and povidone K-90 for 2 to 5 minutes, then add pregelatinized starch and mix for 5 minutes, then add one-fifth of the raw materials and mix for 8 minutes; then add the remaining prescription amount The raw materials were mixed for 12min.
[0055] c. Add the above mixture into the wet granulator, start stirring (400rpm), add purified water with 50% of the total weight of raw and auxiliary materials, cut and make soft materials, and the obtained granules are granulated by a conical granulator;
[0056] d. Fluidized bed drying (55°C, air intake frequenc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com