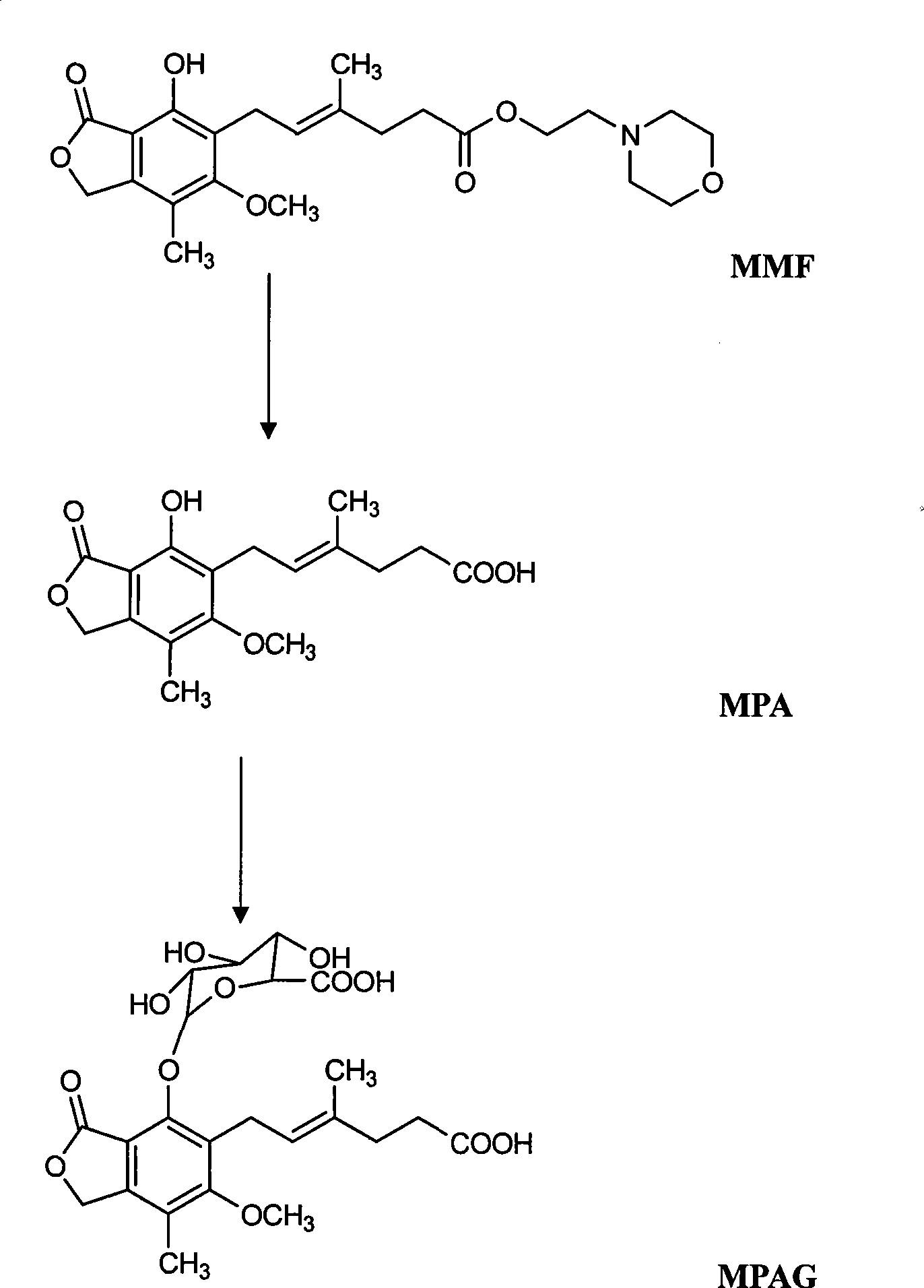
Method for simultaneously determining mycophenolic acid ester, mycophenolic acid and metabolite thereof in human blood plasma
A technology of mycophenolate mofetil and mycophenolate mofetil, applied in the field of medical testing, can solve the problems that have not been seen yet, and achieve the effects of simple operation, low cost and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
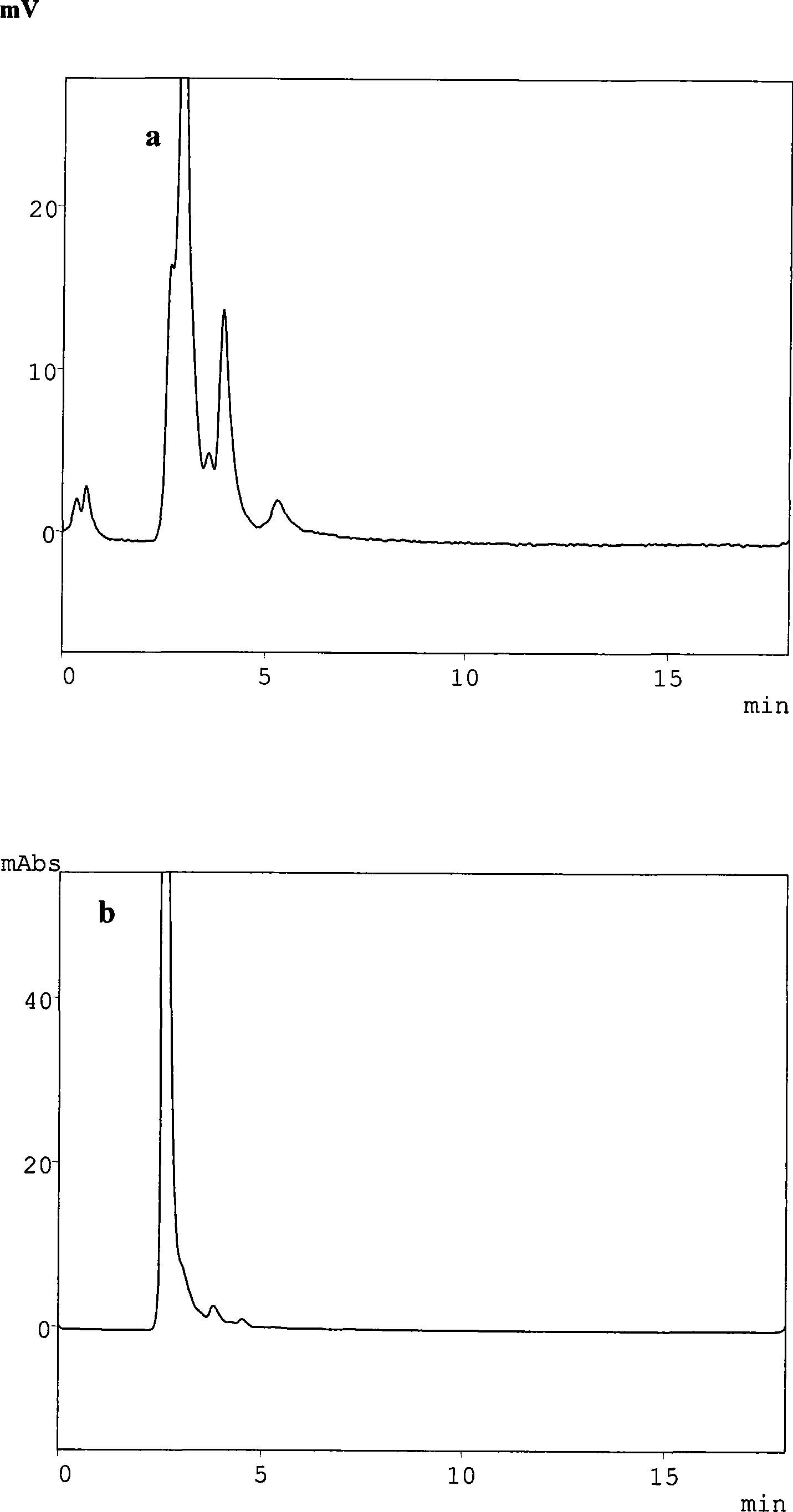
[0028] Embodiment 1: Volunteer plasma MMF, MPA and MPAG are measured
[0029] Chromatographic conditions
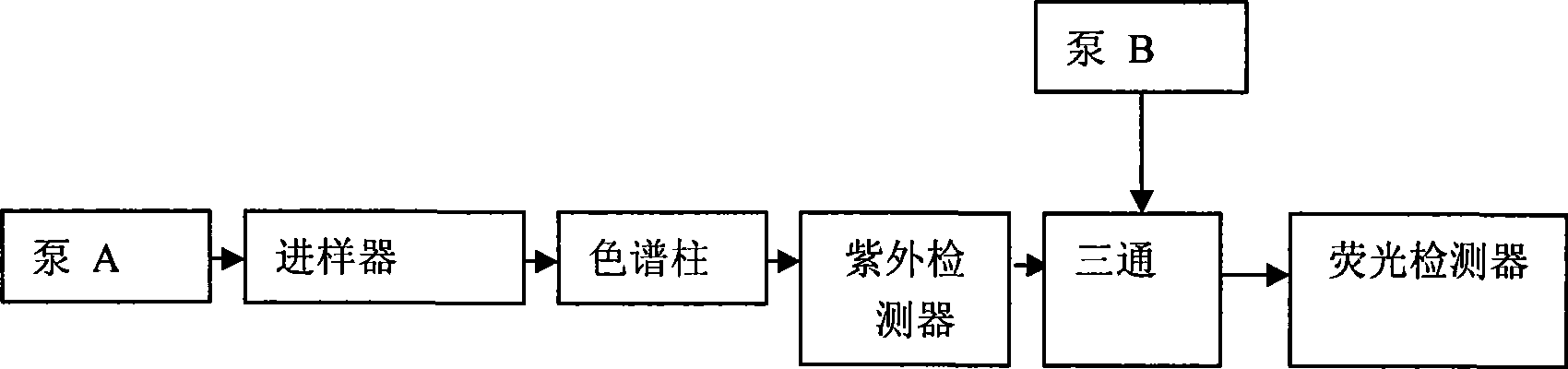
[0030] HPLC system: Japan Shimadzu LC-10A high performance liquid phase instrument (system includes LC-10AD pump, LC-10AT pump, SPD-10A ultraviolet detector, RF-10AXL fluorescence detector, SIL-10A automatic sampler, CBM -10A system communicator, column thermostat, Class-LC10 Version 1.63 chromatographic workstation); chromatographic column: Kromasil-C18 (150mm×4.6mm, 5μm); mobile phase: isocratic elution, methanol——0.1% TFA ( 45:55, V / V); flow rate: 1.2ml / min; column temperature 40°C; fluorescence excitation wavelength 342nm, emission wavelength 425nm.
[0031] Plasma sample pretreatment
[0032] Precisely draw 100 μl of plasma into a 1.5ml centrifuge tube, add 200 μl of acetonitrile solution containing internal standard propafenone 150 μg / ml, vortex for 30 s, centrifuge for 10 min (20,627×g, 4°C), take 20 μl of the supernatant for injection, The internal standard met...
Embodiment 2
[0042] Example 2: Determination of MMF, MPA and MPAG in Plasma of Kidney Transplantation Patients
[0043] Chromatographic conditions
[0044] HPLC system: Japan Shimadzu LC-10A high performance liquid phase instrument (system includes LC-10AD pump, LC-10AT pump, SPD-10A ultraviolet detector, RF-10AXL fluorescence detector, SIL-10A automatic sampler, CBM -10A system communicator, column thermostat, Class-LC10 Version 1.63 chromatographic workstation); chromatographic column: ZORBAX RX-C8 (250mm×4.6mm, 5μm); mobile phase: isocratic elution, methanol——0.1% TFA (52:48, V / V); flow rate: 1.2ml / min; column temperature 45°C; fluorescence excitation wavelength 344nm, emission wavelength 427nm;
[0045] Plasma sample pretreatment
[0046] Precisely draw 100 μl of plasma into a 1.5ml centrifuge tube, add 200 μl of methanol solution containing internal standard propafenone 150 μg / ml, vortex for 30 s, centrifuge for 10 min (20,627×g, 4°C), take 20 μl of the supernatant for injection, T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com