Mycophenolate mofetil lyophilized powder injection for injection and preparation method thereof
A technology of mycophenolate mofetil and freeze-dried powder injection, which is applied in the field of pharmaceutical preparations, can solve the problems of poor reconstitution effect, incomplete freeze-drying, and decreased blood pressure, and achieves the advantages of avoiding nausea, stable quality, and reducing toxic and side effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 0.5g mycophenolate mofetil for injection freeze-dried powder injection
[0033] 1. Prescription composition (prescription volume of 1000 bottles):
[0034] Mycophenolate Mofetil 500g
[0035] Polyethylene glycol-12-hydroxystearate 200g
[0036] Water for injection 6000mL
[0037] Fill in 20mL colorless control bottles to make 1000 bottles.
[0038] 2. Preparation:
[0039] 1) In the full amount of 80% water for injection, add polyethylene glycol-12-hydroxystearate, stir to dissolve, then add mycophenolate mofetil, and then add 2mol / L hydrochloric acid under stirring until Until the mycophenolate mofetil is dissolved; the pH value is adjusted to 3.5 with 2mol / L hydrochloric acid, and the water for injection is added to the full amount to prepare the medicinal solution;
[0040] 2) Add 0.05% (g / mL) wet activated carbon, stir and adsorb for 30min, and then filter with a 0.45μm polypropylene microporous membrane;
[0041] After the filtrate passed the inspec...
Embodiment 2
[0048] Example 2 1.0 g of mycophenolate mofetil for injection freeze-dried powder injection
[0049] 1. Prescription composition (prescription volume of 1000 bottles):
[0050] Mycophenolate Mofetil 1000g
[0051] Polyethylene glycol-12-hydroxystearate 400g
[0052] Water for injection 6000mL
[0053] Fill in 20mL colorless control bottles to make 1000 bottles.
[0054] 2. Preparation:
[0055] 1) Add polyethylene glycol-12-hydroxystearate to the full amount of 80% water for injection, stir to dissolve, then add mycophenolate mofetil, and then add 1mol / L hydrochloric acid to the mofetil under stirring Until the mycophenolate mofetil is dissolved, the pH value is adjusted to 4.5 with 2mol / L sodium hydroxide, and the water for injection is added to the full amount to obtain a medicinal solution;
[0056] 2) Add 0.05% (g / mL) wet activated carbon, stir and adsorb for 30min, and then filter with a 0.45μm microporous membrane (mixed cellulose acetate membrane);
[0057] After ...
Embodiment 3
[0064] Embodiment 3 influence factor test
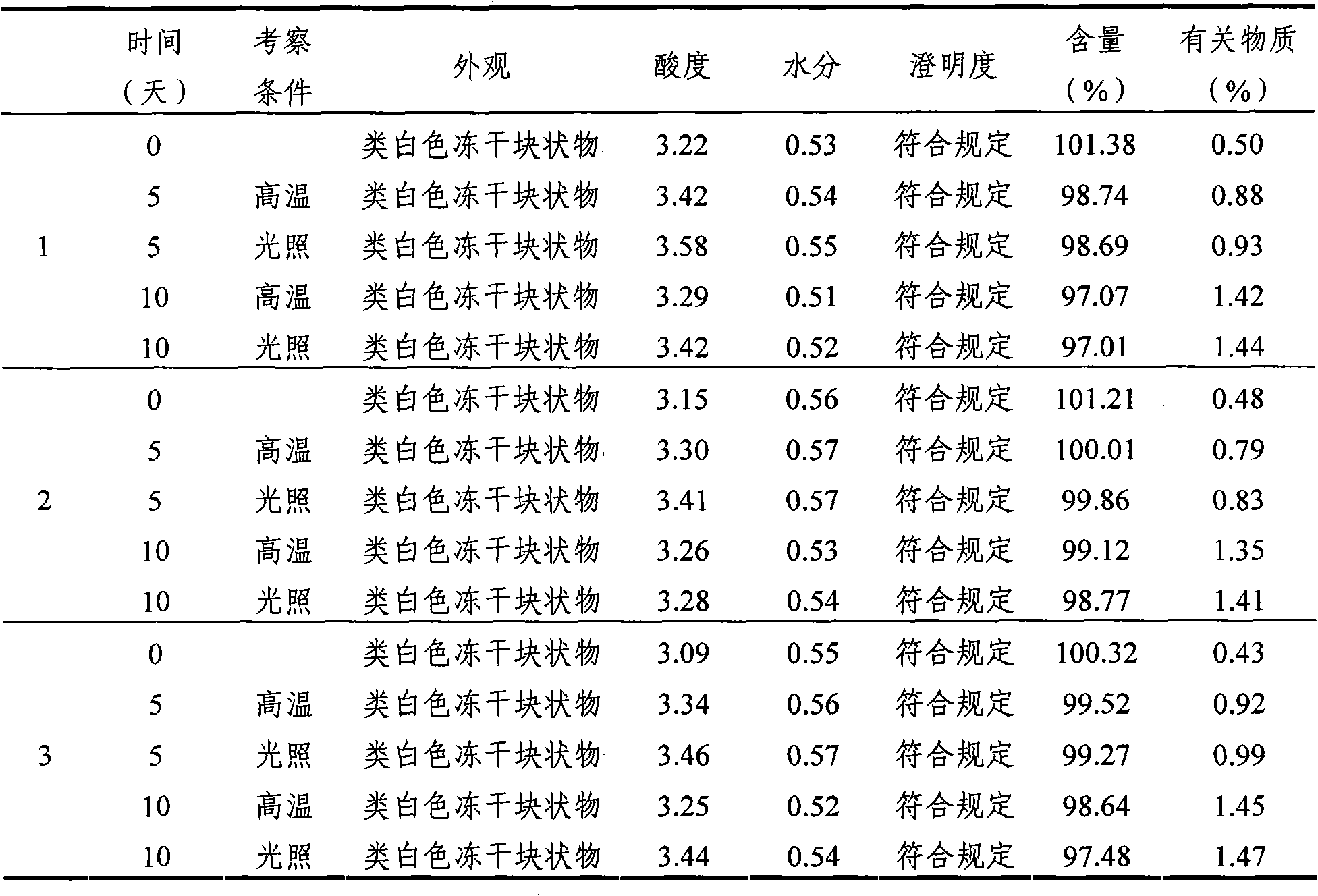
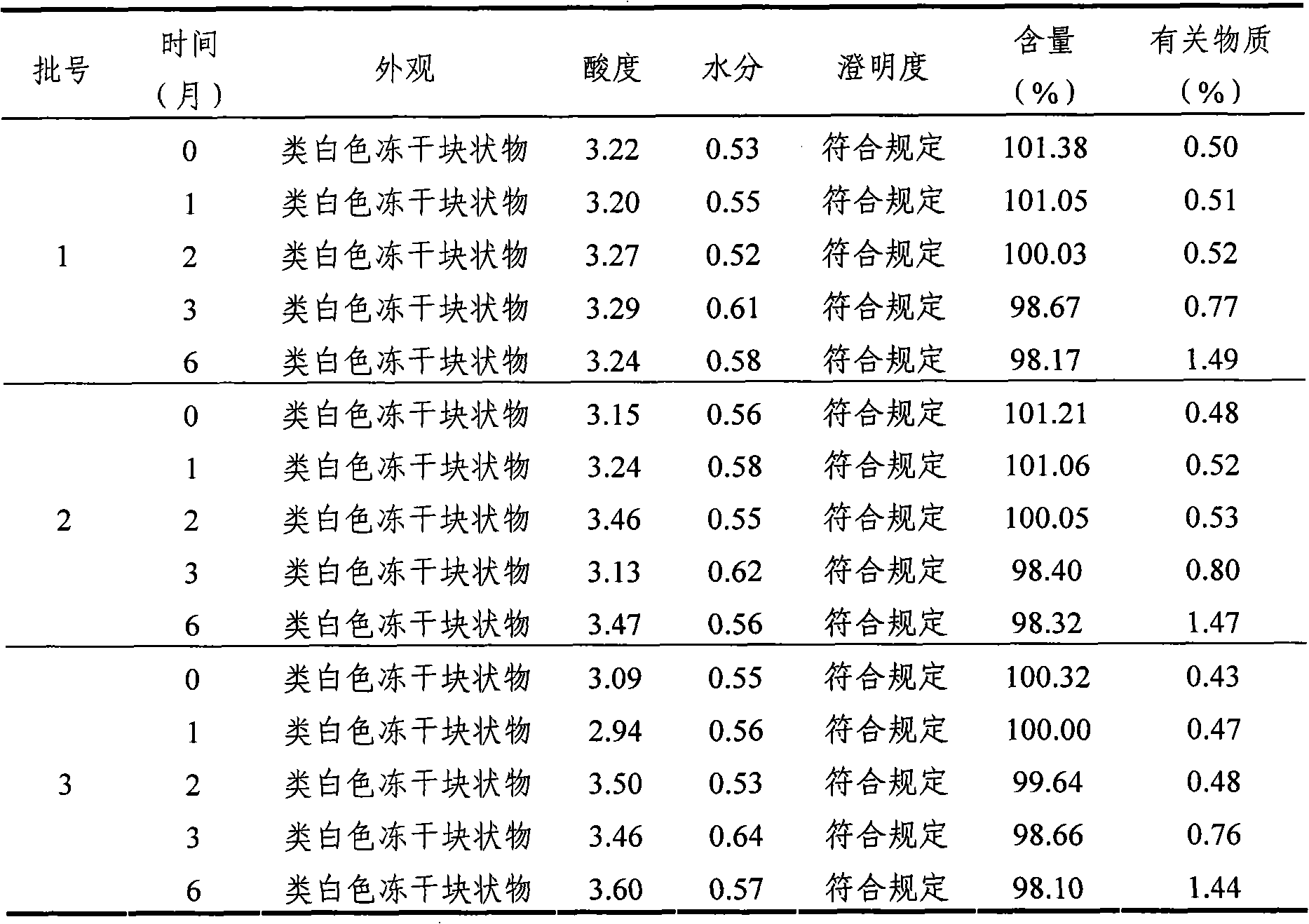
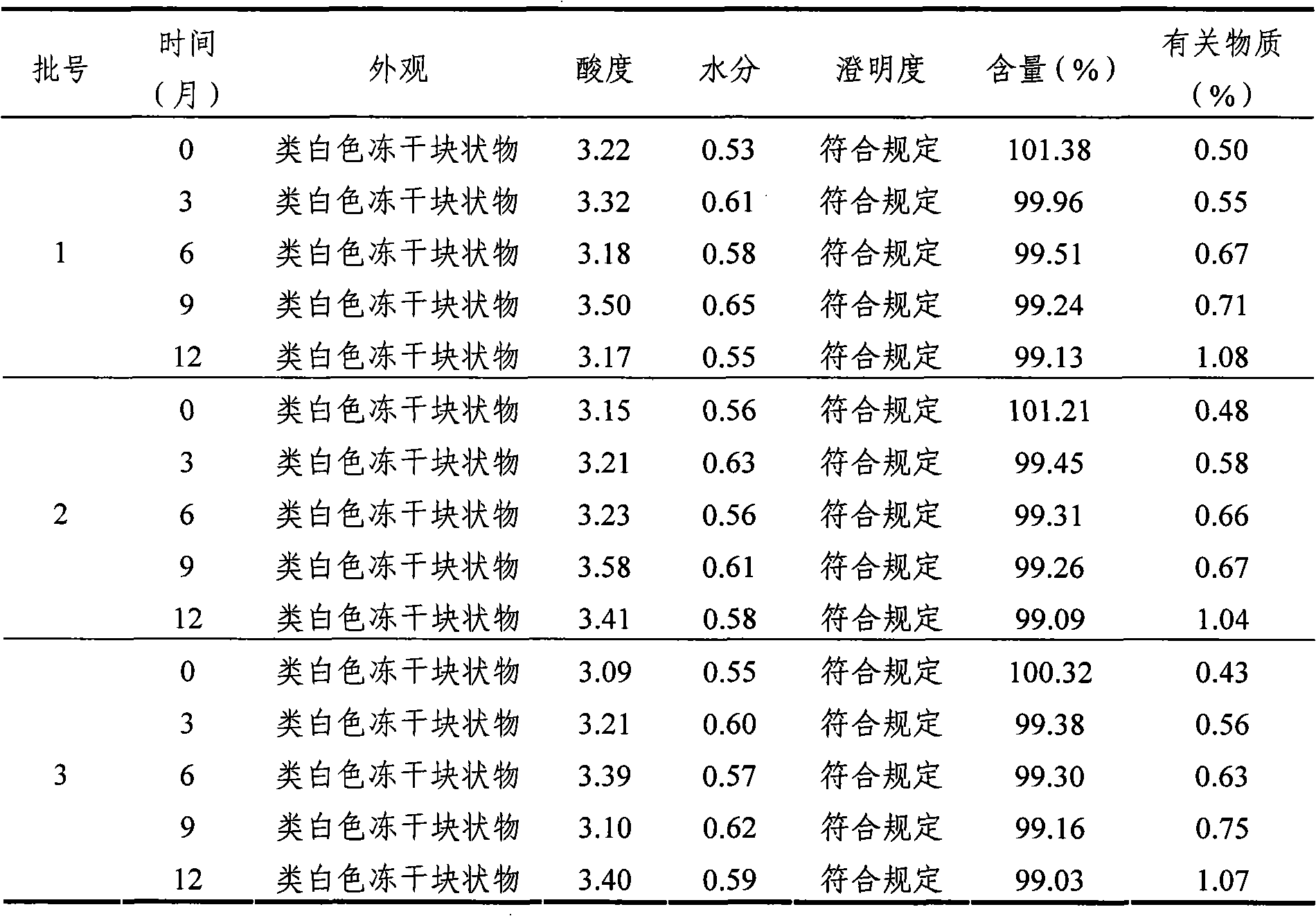
[0065] According to the requirements of Appendix X IX C (Guiding Principles for Drug Stability Tests) of Part Two of the Chinese Pharmacopoeia (2005 Edition) and the "Key Items Table for Stability Inspection of Raw Materials and Pharmaceutical Preparations" in "Guiding Principles for Research on Chemical Drugs and Therapeutic Biological Products", The mycophenolate mofetil mofetil injection freeze-dried powder for injection of Example 1 is subjected to the influence factor test, focusing on the sample appearance, acidity, moisture, clarity, content and related substances. The specific process is as follows:
[0066] Take three batches of mycophenolate mofetil mofetil injection freeze-dried powder injection of Example 1, according to the commercially available package, carry out high temperature (60 ℃) and strong light irradiation (4500 ± 500LX) test, measure according to the above-mentioned key investigation items. The results are sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com