Improved enteric medicine composition with mycophenolate
A technology of mycophenolate mofetil and enteric coating, which is applied in the field of pharmaceutical compositions coated with enteric coating, can solve the problems of improvement, and achieve the effect of excellent drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
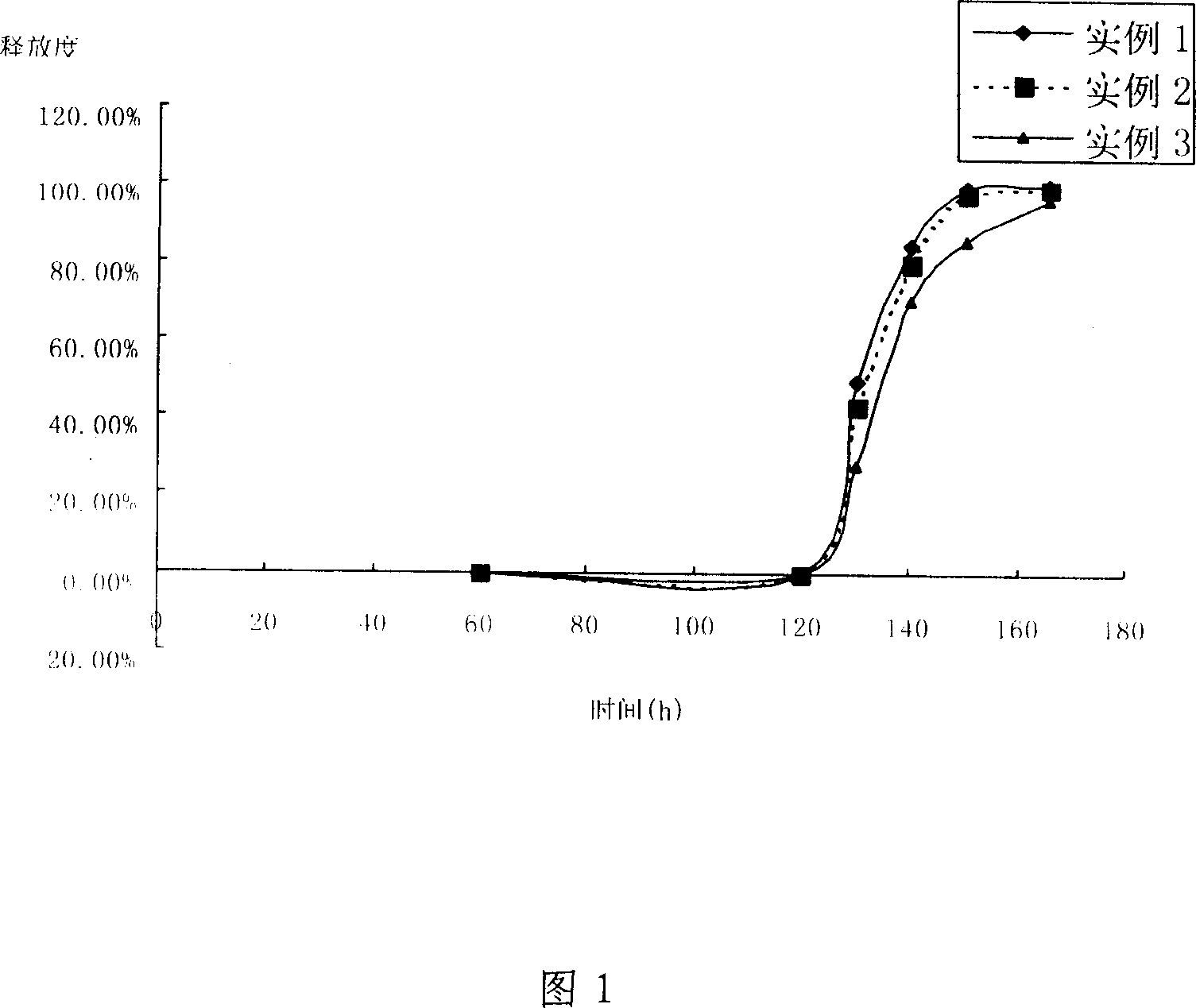
Image
Examples
Embodiment 1
[0030] Tablet core prescription:
[0031] Mycophenolate sodium
180g (based on mycophenolic acid)
40g
Crosslinked PVP
12.5g
PVP K30 Aqueous solution
Right amount
1g
production
1000 pieces
[0032] Coating liquid prescription:
[0033] Opadry OY-P type
200g
85% ethanol
1000ml
production
1000ml
[0034] Preparation method: Weigh the prescribed amount of mycophenolate sodium, lactose, and cross-linked PVP, mix well, and use PVP K30 The aqueous solution is granulated, dried, magnesium stearate is added in the prescribed amount, and the tablet core is obtained by pressing after mixing.
[0035] Take the prescription amount of Opadry OY-P and disperse it in the prescription amount of 85% ethanol, and then coat it to increase the weight by about 10%.
Embodiment 2
[0037] Tablet core prescription:
[0038] Mycophenolate sodium
180g (based on mycophenolic acid)
40g
Crosslinked PVP
12.5g
PVP K30 Aqueous solution
Right amount
1g
production
1000 pieces
[0039] Coating liquid prescription:
[0040] Hydroxypropyl methylcellulose acetate succinate
200g
Titanium dioxide
50g
80% ethanol
2000ml
production
2000ml
[0041] Preparation method: Weigh the prescribed amount of mycophenolate sodium, lactose, and cross-linked PVP, mix well, and use PVP K30 The aqueous solution is granulated, dried, magnesium stearate is added in the prescribed amount, and the tablet core is obtained by pressing after mixing.
[0042] Dissolve the prescription amount of coating material in 80% ethanol of the prescription amount, and then grind the titanium dioxide to make a coating solution,...
Embodiment 3
[0044] This embodiment provides tablets made according to the relevant parameters provided in the invention patent of CN1104238C.
[0045] Tablet core prescription:
[0046] Mycophenolate sodium
180g (based on mycophenolic acid)
40g
Crosslinked PVP
12.5g
PVP K30 Aqueous solution
Right amount
Magnesium stearate
1g
production
1000 pieces
[0047] Coating liquid prescription:
[0048] Acrylic resin No. 2
250g
100g
75g
Tween 80
35g
Titanium dioxide
50g
95% ethanol
3000ml
production
3000ml
[0049] Preparation method: Weigh the prescribed amount of mycophenolate sodium, lactose, and cross-linked PVP, mix well, and use PVP K30 The aqueous solution is granulated, dried, magnesium stearate is added in the prescribed amount, and the tablet core is ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com