Compound preparation for treating immunological rejection after organ transplantation and preparation method thereof
A compound preparation and immune rejection technology, which is applied in the field of compound preparation and its preparation for the treatment of immune rejection after organ transplantation, can solve the problems affecting the physical and mental health of patients with curative effects, and achieve the effect of avoiding gastrointestinal adverse reactions and weakening side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
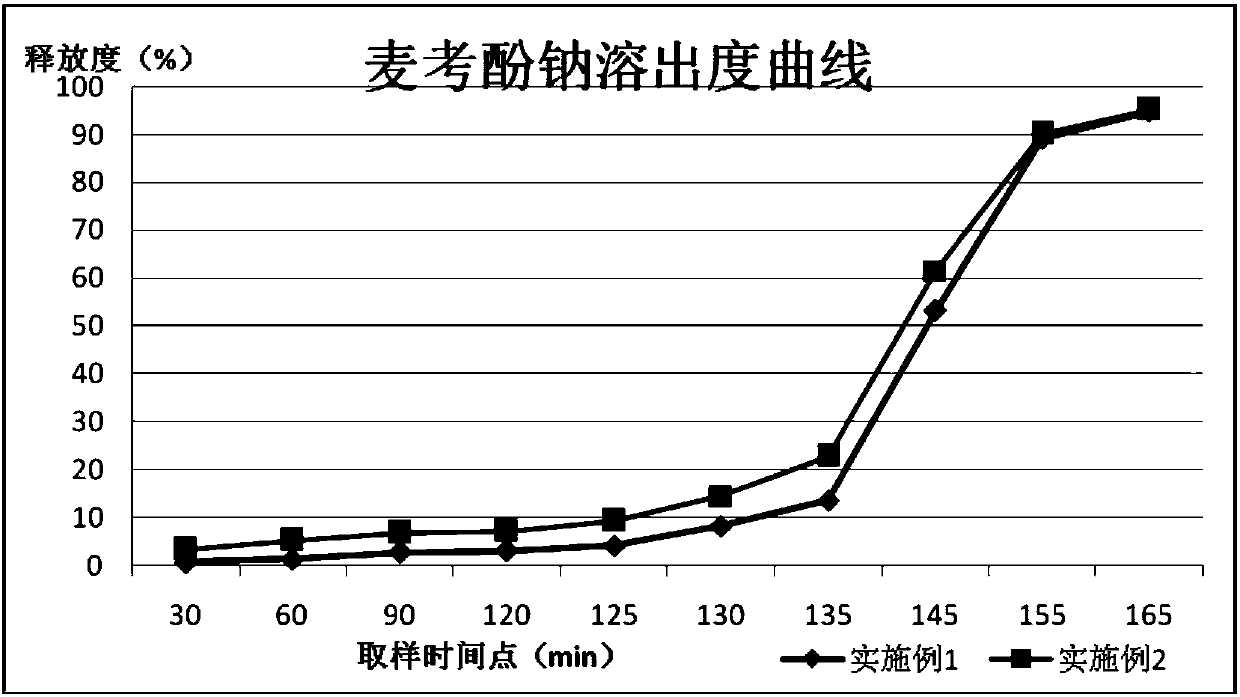
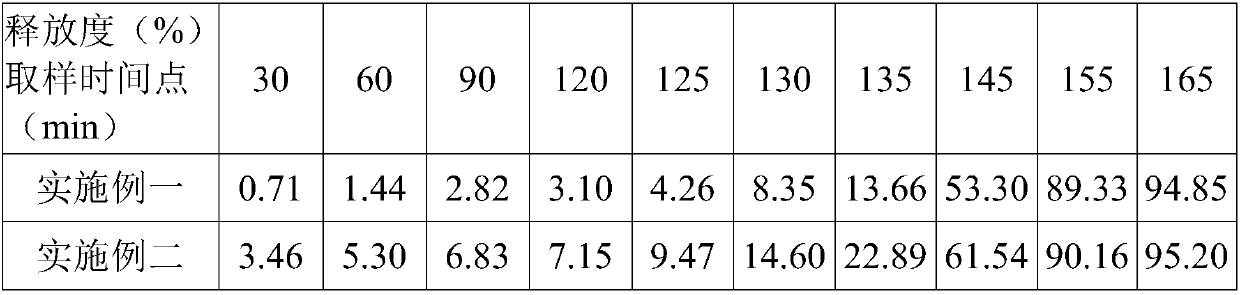
[0027] This example discloses the first specific implementation of a compound preparation for treating immune rejection after organ transplantation. The compound preparation is specifically a capsule, and its prescription is as follows:
[0028] Mycophenolate sodium 1500g, anhydrous lactose (filler) 352g, povidone K30 (binder) 220g, starch (diluent) 132g, cross-linked povidone (disintegrant) 352g, colloidal silicon dioxide (Glidant) 60g, magnesium stearate (lubricant) 30g, coating material 352g, salvia miltiorrhiza 440g, chuanxiong 350g, angelica 175g, astragalus 175g, tripterygium wilfordii 85g, safflower 85g.
[0029] A total of 10,000 capsules were made.
[0030] The preparation method of this compound preparation is as follows.
[0031] Pass the mycophenolate sodium, filler, disintegrant and other raw materials in the prescription quantity through a 100-mesh sieve, and set aside.
[0032] Step (1): After sieving mycophenolate sodium, filler, disintegrant, and diluent, p...
Embodiment 2
[0040] This example discloses the first specific implementation of a compound preparation for treating immune rejection after organ transplantation. The compound preparation is specifically a tablet, and its prescription is as follows:
[0041] Mycophenolate Sodium 1750g, Anhydrous Lactose 380g, Povidone K30 230g, Starch 134g, Crospovidone 346g, Colloidal Silicon Dioxide 65g, Magnesium Stearate 35g, Coating Material 360g, Salvia miltiorrhiza 400g, Chuanxiong 360g , Angelica 180g, Astragalus 180g, Tripterygium wilfordii 80g, safflower 80g.
[0042] A total of 10000 tablets were made.
[0043] The preparation method of this compound preparation is as follows.
[0044] Pass the mycophenolate sodium, filler, disintegrant and other raw materials in the prescription quantity through a 100-mesh sieve, and set aside.
[0045] Step (1): After sieving mycophenolate sodium, filler, disintegrant, and diluent, put them into a wet granulator for premixing, and add a prescribed amount of bi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com