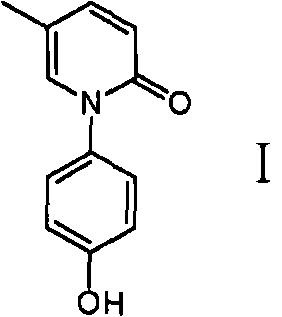
Method for preparing oxycodone
A technology of dehydroxylation and methylpyridone is applied in the field of preparation of oxynidone, which can solve the problems of easy formation of highly toxic gas, complicated operation, complicated sewage and waste treatment and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
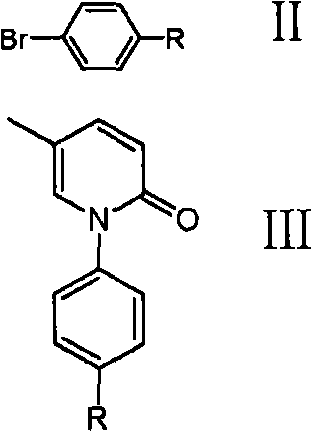
[0038] The preparation method of the compound of formula I provided by the present invention is to mix 5-methylpyridone (compound of formula 4, or compound 4) and compound of formula II to obtain compound of formula III, and then react with dehydroxylation protection reagent to obtain formula I Compound:
[0039]
[0040] Wherein the R of formula II compound is an ether group, is selected from benzyl ether (-OCH 2 C 6 h 5 ), cyclohexyl ether tert-butyl ether (-OC(CH 3 ) 3 ), 4-picolyl ether or tetrahydropyranyl ether (-OTHP or
[0041] 5-picoline and formula II compound reaction obtains formula III compound, can carry out under the condition of this area routine, in a preferred example of the present invention is in dimethylformamide (DMF), anhydrous potassium carbonate and in the presence of cuprous iodide:
[0042]
[0043] In the present invention, according to the difference of R in the compound of formula III, in the step of obtaining the compound of for...
preparation example 1
[0072]
[0073] In 15mL of DMF, add 5g of 5-methylpyridone (compound 4), 17.7g of p-bromobenzenetetrahydropyranyl ether (compound 2, purchased from Sigma), 7.6g of anhydrous K 2 CO 3 , 1.06g CuI, the mixture was heated to 140 degrees, and the reaction was stirred for 5 hours. The completion of the reaction was monitored by TCL, cooled to room temperature, and filtered. The filter residue was washed with DMF, the filtrates were combined, and the solid was discarded. DMF was distilled off under reduced pressure, the residue was dissolved in ethyl acetate, washed with water, and then washed with saturated brine, the organic phase was dried over anhydrous sodium sulfate, concentrated, and dried to obtain an off-white to light gray solid, that is, compound 5 (9.7 g, recovered rate of 74%).
[0074] 5 g of compound 5 was dissolved in 30 mL of ethanol, and 0.05 g of p-toluenesulfonic acid was added. Stir at room temperature for about 1 hour. TLC showed that the reaction was c...
preparation example 2
[0077]
[0078] Compound 7 (yield 69%) was prepared by basically the same method as in Example 1, except that p-bromobenzenetetrahydropyranyl ether (compound 2) was replaced by bromophenylbenzyl ether (compound 6).
[0079] 5 g of compound 7 was dissolved in 30 mL of tetrahydrofuran, hydrogen gas was introduced, and 0.5 g of 10% Pd / C was added. Stir at room temperature for about 5 hours. TLC showed that the reaction was complete, filtered, and the filtrate was concentrated. 15 ml of ethanol was added to the residue, recrystallized, and filtered to obtain the compound of formula I (2.7 g, 78%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com