Opioid ketal compounds and uses thereof
a technology of opioid ketal and ketal compounds, which is applied in the field of new opioid ketal compounds, can solve the problems of abuse and diversion, and the use of opioid compounds has been reported to have a number of potential side effects, and achieve the effects of treating, preventing pain, and preventing pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation examples
Example 1
[0220]
[0221]Preparation of a compound of Formula IV (oxycodone 2,4-pentanediol ketal): Oxycodone free base (2.91 g), p-toluenesulfonic acid monohydrate (2.17 g) and 2,4-pentanediol (5.40 g, mixture of isomers) were stirred in toluene (250 mL) and heated under reflux with a Dean Stark water trap attached. After 3½ hours, the mixture was cooled, treated with triethylamine (5 mL), and washed with water (2×50 mL). The toluene solution was concentrated under reduced pressure to a clear resin that solidified on standing to afford a white solid (Formula I) (3.87 g). FIG. 7 provides the 1H NMR (d6-DMSO) spectrum of the compound of Formula IV (oxycodone 2,4-pentanediol ketal).
[0222]Using the procedure detailed herein, isomers IVC and IVD were prepared by reacting oxycodone with 2S,4S-pentanediol and 2R,4R-pentanediol, respectively. In addition, using the procedure detailed herein, a mixture of isomers IVA and IVB was prepared by reacting oxycodone with meso-2,4-pentanediol.
example 2
[0223]
[0224]Preparation of compound of Formula V (oxycodone 1,3-butanediol ketal): Oxycodone free base (1.58 g), p-toluenesulfonic acid (1.19 g) and 1,3-butanediol (5.73 g, mixture of isomers) were stirred in toluene (125 mL) and heated under reflux with a Dean Stark water trap attached. After 5 hours the mixture was cooled and washed with saturated sodium bicarbonate solution (2×50 mL), then with water (50 ml). The solution was dried over magnesium sulfate, filtered, and concentrated under reduced pressure to a colorless resin that solidified on standing to afford a white solid (2.12 g). FIG. 8 provides the 1H NMR (d6-DMSO) spectrum of the compound of Formula V (oxycodone 1,3-butanediol ketal).
[0225]Using the general scheme shown above, the following compounds were prepared and characterized. Characterization was carried out using an LC / MS system. The LC / MS utilized a Phenomenex Luna C18 column and a gradient elution with the first solvent of 90% 2.8 mM ammonium formate in water, 1...
example 3
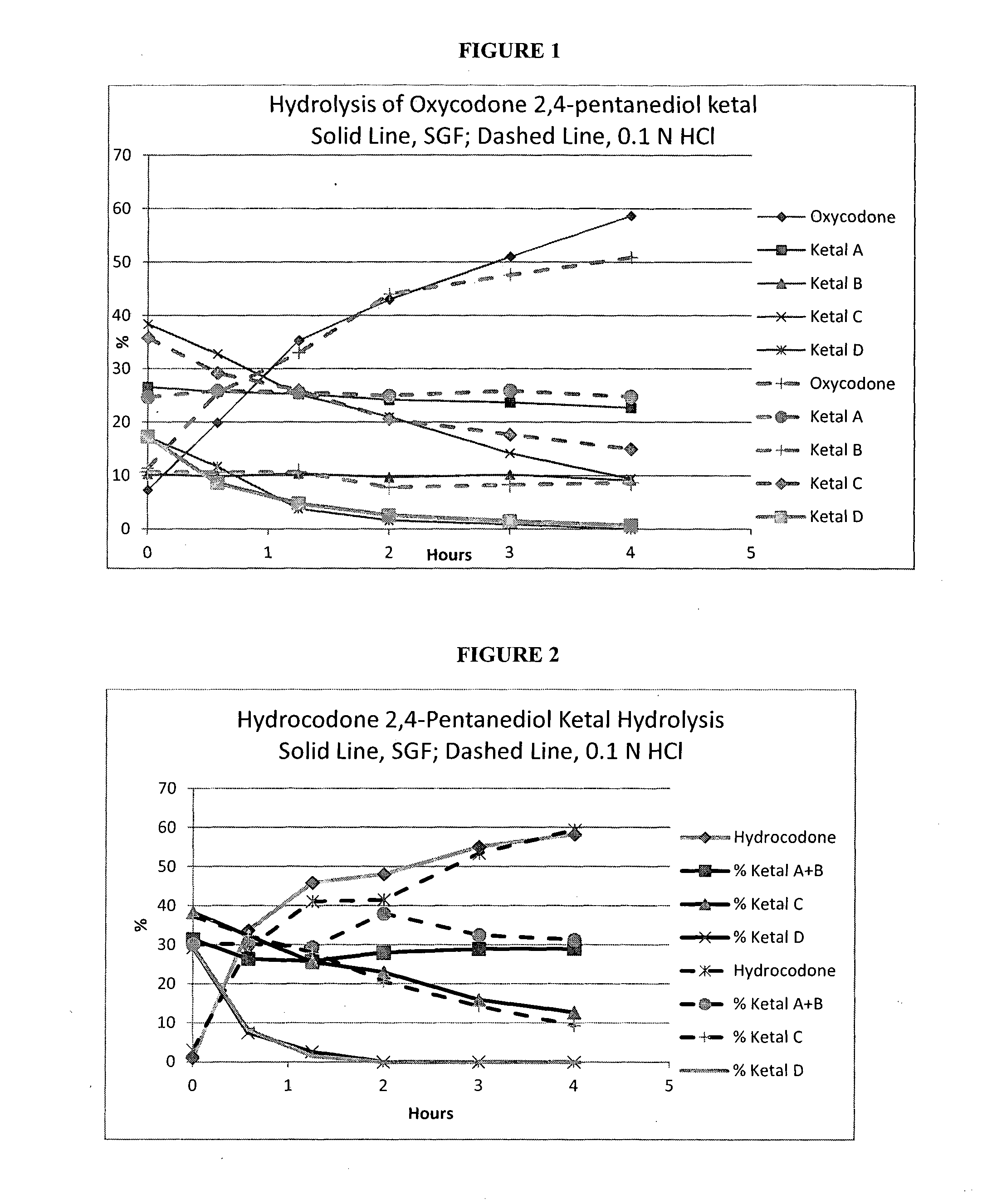
[0227]A mixture of isomers of oxycodone 2,4-pentanediol ketal along with unreacted oxycodone at a concentration of 2 mg / ml was subjected to hydrolysis in USP Simulated Gastric Fluid (SGF) (0.2% NaCl and 0.32% pepsin in 0.084 N HCl) at 37° C., with analysis of the hydrolyzed oxycodone conducted by LC / MS. Results from the hydrolysis are shown in Table 1a and illustrated in FIG. 1.
[0228]As a comparison, a mixture of isomers of oxycodone 2,4-pentanediol ketal along with unreacted oxycodone was dissolved in 0.1 N HCl at a concentration of 2 mg / ml and heated to 37° C. The course of the hydrolysis was monitored by LC / MS. Results from the hydrolysis are shown in Table 1b and illustrated in FIG. 1.
TABLE 1aSimulated Gastric FluidKetalKetalKetalKetalHoursOxycodone ABCD07.326.610.338.417.30.5819.925.79.832.811.71.2535.325.310.425.23.824324.29.7211.635123.710.214.20.85458.722.79.19.40
TABLE 1b0.1N HClKetalKetalKetalKetalHoursOxycodone ABCD011.424.710.735.817.30.58325.525.910.629.28.61.1673325.610...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com