A kind of acyl guanidine compound and its preparation method and application
A compound and drug technology, applied in the field of medicine, can solve problems such as insufficient intestinal absorption, limitation of dosage form and route of administration, low water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048]
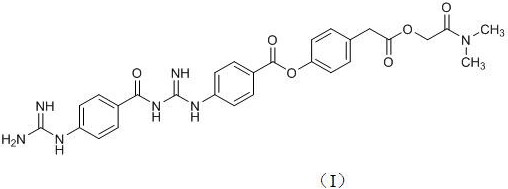
[0049] Add 30g of compound (II), 500ml of acetonitrile, 27g of compound (III), 29g of EDCI (1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride) in the reaction flask, and heat up To reflux, react for 5h. After the reaction was completed, it was evaporated to dryness to obtain an oil. Add 500ml of water, stir vigorously, and filter to obtain the crude compound of formula (IV). The crude product was purified by flash preparative chromatography to obtain 38.2 g of compound (IV).
[0050]
[0051] Add the compound of formula (IV) above into 500ml of acetonitrile, add 27g of compound (III), 29g of EDCI in total, raise the temperature to reflux, and react for 48h. After the reaction was completed, it was evaporated to dryness to obtain an oil. Add 500ml of water, stir vigorously, and filter to obtain the crude compound of formula (I). The crude product was purified by flash preparative chromatography to obtain 45.5 g of compound (I). 1 H NMR: 8.11(d, 2H, ...
Embodiment 2
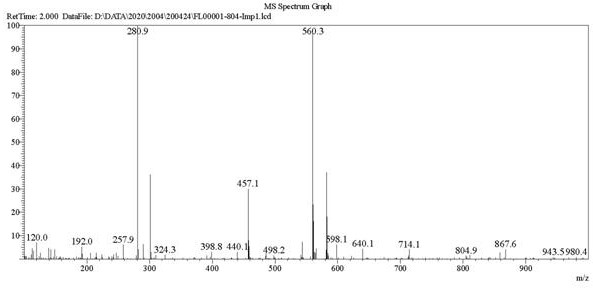
[0053] Add 30g of compound (II), 500ml of toluene, and 58g of EDCI in the reaction bottle, raise the temperature to reflux, add 54g of compound (III) in two batches, and react for 9h. After the reaction was completed, it was evaporated to dryness to obtain an oil. Add 1000ml of water, stir vigorously, and filter to obtain the crude compound of formula (I). The crude product was purified by flash preparative chromatography to obtain 49.3 g of compound (I). 1 H NMR: 8.11(d, 2H, J =8.8Hz), 7.96(d, 2H, J =8.4Hz), 7.85(d, 2H, J =84Hz), 7.39(d, 2H, J =8.4Hz),7.23(dd, 2H, J =2Hz, 6.4Hz), 6.84(d, 2H, J =8.4Hz), 5.44(br s), 4.82(s, 2H), 3.81(s, 2H), 2.91(s, 3H), 2.91(s, 3H). ESI-MS: 560.3.
Embodiment 3
[0055]
[0056] Add 100ml of thionyl chloride and 27g of compound (III) into the reaction flask, stir at reflux for 4h, evaporate to dryness, entrain with toluene, and remove thionyl chloride. Add 500ml of dichloromethane, 27g of compound (III), and reflux for 12h. Evaporated to dryness, the oil (V) was obtained, which was continued to the next step without treatment.
[0057]
[0058] Add 30g of compound (II) and 29g of EDCI to tetrahydrofuran (V) solution obtained above, reflux for 48h, stop the reaction, evaporate to dryness, add 1000ml of water and stir vigorously, filter to obtain the crude compound of formula (I). The crude product was purified by flash preparative chromatography to obtain 52.8 g of compound (I). 1 H NMR: 8.11(d, 2H, J =8.8Hz), 7.96(d, 2H, J =8.4Hz),7.85(d, 2H, J =84Hz), 7.39(d, 2H, J =8.4Hz), 7.23(dd, 2H, J =2Hz, 6.4Hz), 6.84(d, 2H, J =8.4Hz), 5.44(br s), 4.82(s, 2H), 3.81(s, 2H), 2.91(s, 3H), 2.91(s,3H). ESI-MS: 560.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com