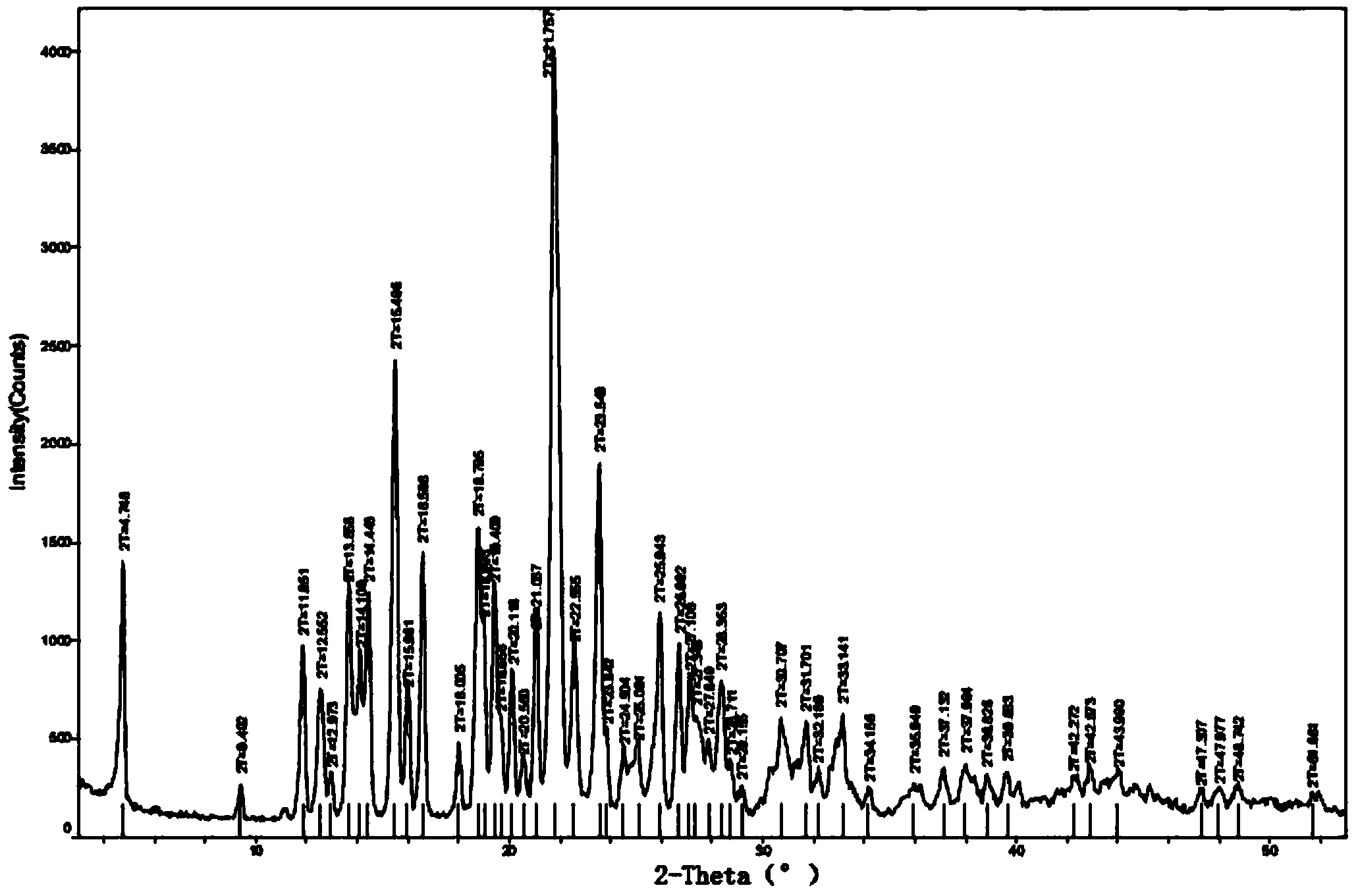
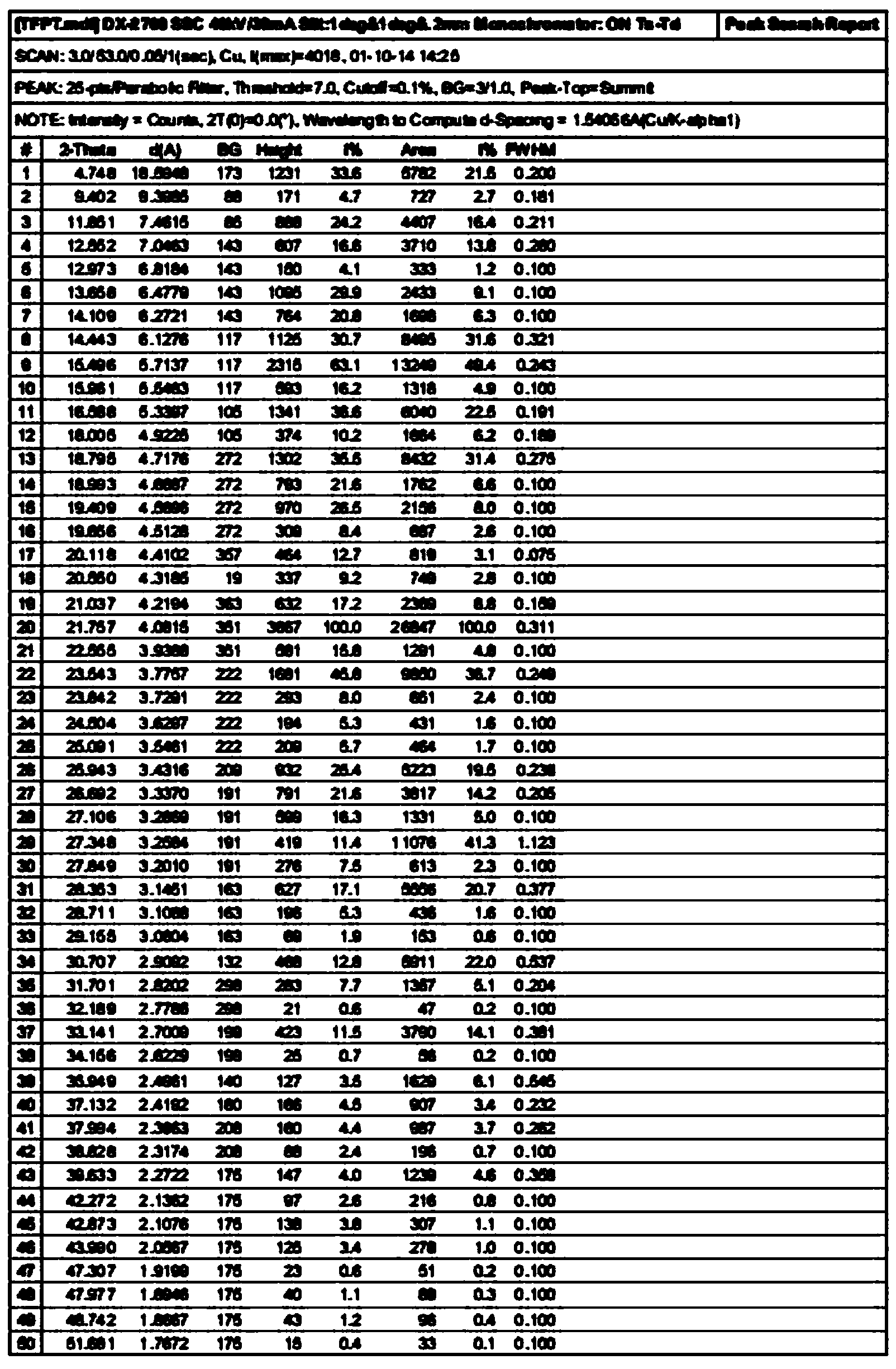
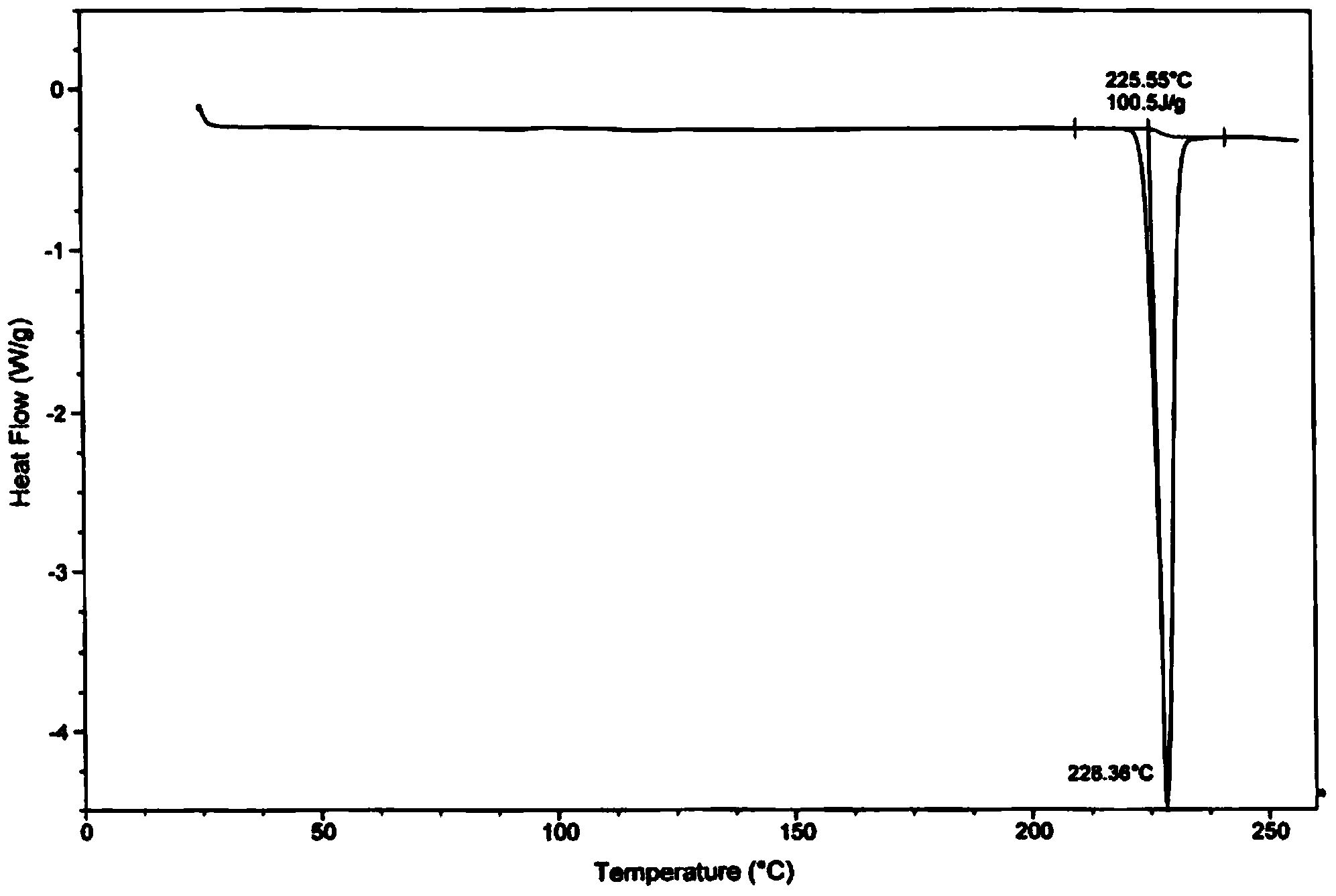
Method for preparing amorphous tolvaptan
A tolvaptan and amorphous technology, applied in the field of preparation of amorphous tolvaptan, can solve the problems of low yield, long time consumption, unfavorable preparation and promotion, etc., and achieve simple preparation method, easy operation, high The effect of clinical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 1500mL of acetone and 50g of tolvaptan crystalline compound into a 2L reaction flask, heat and stir until completely dissolved to obtain an acetone-tolvaptan solution, and naturally cool to 25°C under stirring for later use.
[0032] Add 6000mL of pre-cooled n-hexane (temperature 2°C) to the 10L reaction flask, and quickly add the above-mentioned acetone-tolvaptan solution dropwise to n-hexane within 5 minutes under rapid stirring, and a large amount of solids are precipitated immediately. After completion, continue stirring for 15 minutes, filter, and dry under reduced pressure at 60° C. to constant weight to obtain 47.5 g of amorphous tolvaptan, with a yield of 95%.
Embodiment 2
[0034] Add 500mL of methanol and 50g of tolvaptan crystalline compound into a 1L reaction flask, heat and stir until completely dissolved to obtain a methanol-tolvaptan solution, and naturally cool to 35°C under stirring for later use.
[0035] Add 1500mL of pre-cooled water (at a temperature of 5°C) to the 3L reaction bottle, pour it into the above tolvaptan solution under rapid stirring, and immediately precipitate a large amount of solid, after pouring, continue to stir for 5 minutes, filter, 50°C Drying under reduced pressure to constant weight yielded 45.8 g of amorphous tolvaptan, with a yield of 91.6%.
Embodiment 3
[0037] Add 800mL of ethanol and 50g of tolvaptan crystal form compound into a 2L reaction flask, heat and stir until completely dissolved to obtain an ethanol-tolvaptan solution, and naturally cool to 40°C under stirring for later use.
[0038] Add 2000mL of pre-cooled water (at a temperature of 4°C) to the 5L reaction bottle, and quickly add the above ethanol-tolvaptan solution dropwise within 5 minutes under rapid stirring, and a large amount of solids are precipitated immediately. After the dropwise addition, continue to Stir for 15 minutes, filter, and dry under reduced pressure at 60° C. to constant weight to obtain 47 g of amorphous tolvaptan, with a yield of 94%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com