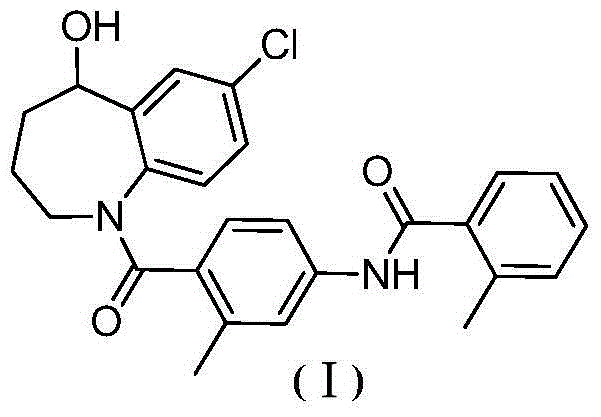
Preparation method for cardiovascular disease treatment drug
A technology of compounds and inert solvents, which is applied in the field of preparation of drugs for treating cardiovascular diseases, can solve problems such as unfavorable industrial operation and economical industrial production, difficulty in obtaining starting materials, and increased production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0104] The preparation of formula (VI) compound
[0105] The present invention also provides a method for preparing the compound of formula (VI), the method comprising the steps of:
[0106] (2) In an inert solvent, react the compound of formula (IV) with the compound of formula (V) to obtain the compound of formula (VI);
[0107]
[0108] In a preferred embodiment of the present invention, the reaction is carried out in the presence of a base catalyst; preferably, the base catalyst is selected from the group consisting of triethylamine, pyridine, diisopropylethylamine, N- Methylmorpholine, DBU, or combinations thereof; preferably triethylamine, diisopropylethylamine, or combinations thereof.
[0109] In another preferred embodiment, in the step (2), the base catalyst is slowly added to the compound of formula (V) at a suitable temperature.
[0110] In another preferred example, in the step (2), the inert solvent is selected from the group consisting of dichloromethane, a...
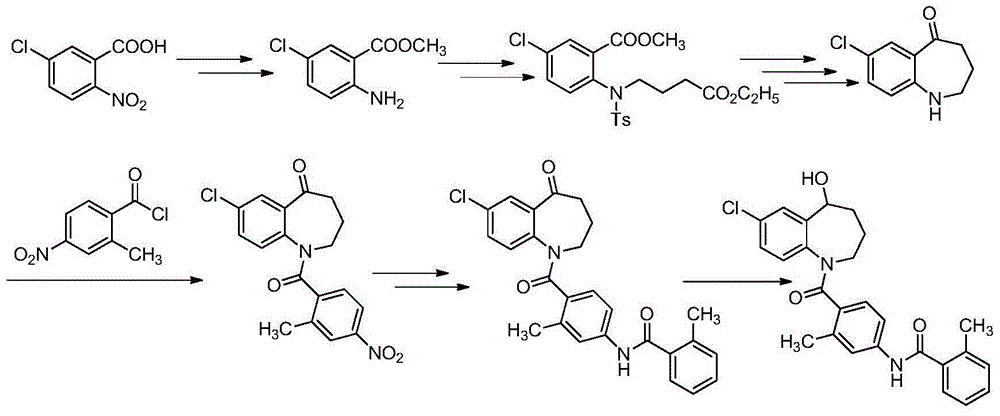
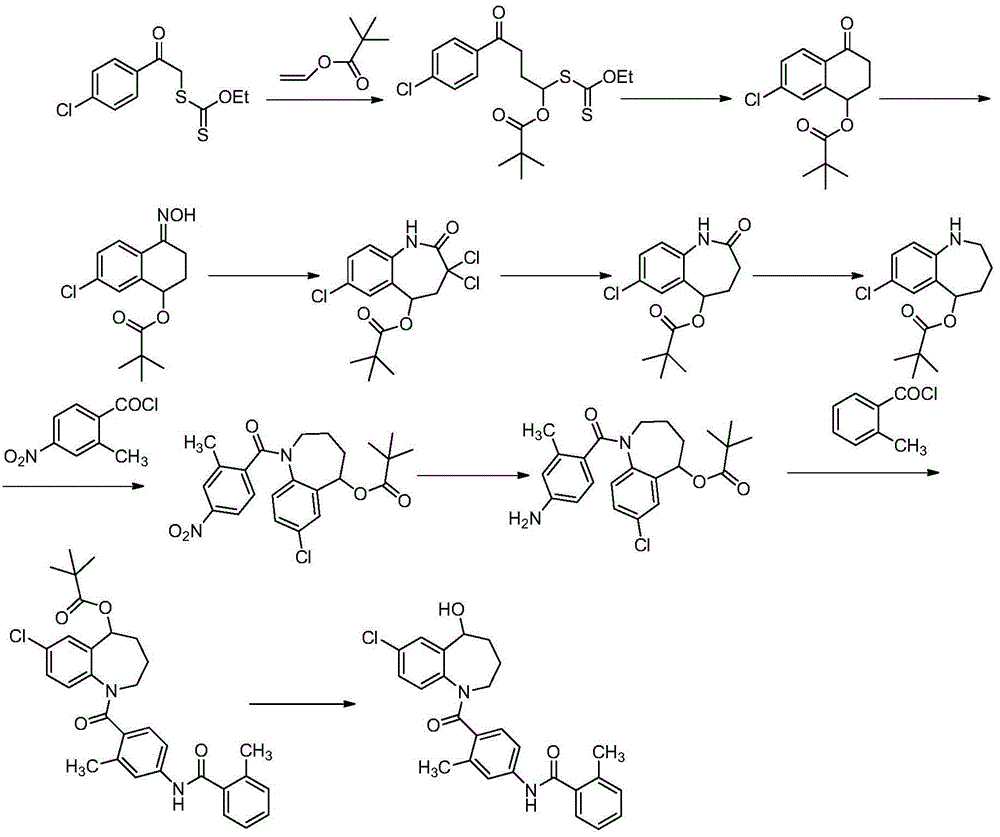
Embodiment 1
[0197] (1) Synthesis of Compound (Ⅳ)
[0198] Methyl 2-amino-5-chlorobenzoate (30.0 g, 161.63 mmol) and methyl 4-bromobutyrate (Ⅲ) (29.26 g, 161.63 mmol) were dissolved in acetonitrile (600 mL), and sodium carbonate ( 34.26g, 323.26mmol), then stirred and heated to 80°C, reacted for 5h, and detected by TLC, the reaction was complete. Cool to room temperature, pour the reaction solution into water (300mL), take the organic phase, extract the aqueous phase with ethyl acetate twice (200mLx2), combine the organic phases, wash with water twice (200mLx2), and dry over anhydrous sodium sulfate. The solvent was spun off to obtain 43.41 g of compound (IV), with a yield of 94.0%.
[0199] 1 H-NMR(400MHz,DMSO):δ7.69(s,1H),7.50(s,1H),6.75(s,1H),6.61(s,1H),3.85(s,3H),3.65(s, 3H), 3.33(m,2H), 2.50(m,2H), 2.03(m,2H). C 13 h 16 ClNO 4 (M+H) + Calcd: 285.0768, found: 285.0771.
[0200] (2) Synthesis of compound (Ⅵ)
[0201] Compound (Ⅳ) (40.0g, 140.00mmol) was dissolved in dichlorome...
Embodiment 2
[0214] (1) Synthesis of Compound (Ⅳ)
[0215]Methyl 2-amino-5-chlorobenzoate (30.0 g, 161.63 mmol) and methyl 4-bromobutyrate (Ⅲ) (29.26 g, 161.63 mmol) were dissolved in acetonitrile (600 mL), and potassium carbonate ( 44.68g, 323.26mmol), then stirred and heated to 80°C, reacted for 5h, and detected by TLC, the reaction was complete. Cool to room temperature, pour the reaction solution into water (300mL), take the organic phase, extract the aqueous phase with ethyl acetate twice (200mLx2), combine the organic phases, wash with water twice (200mLx2), and dry over anhydrous sodium sulfate. The solvent was spun off to obtain 42.49 g of compound (Ⅳ), with a yield of 92.0%.
[0216] (2) Synthesis of compound (Ⅵ)
[0217] Compound (Ⅳ) (40.0g, 140.00mmol) was dissolved in dichloromethane (600mL), diisopropylethylamine (36.19g, 280.00mmol) was added under stirring, and 2-methyl- 4-Nitrobenzoyl chloride (Ⅴ) (33.53g, 168.00mmol), reacted for 4h, and TLC detected that the reaction w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com