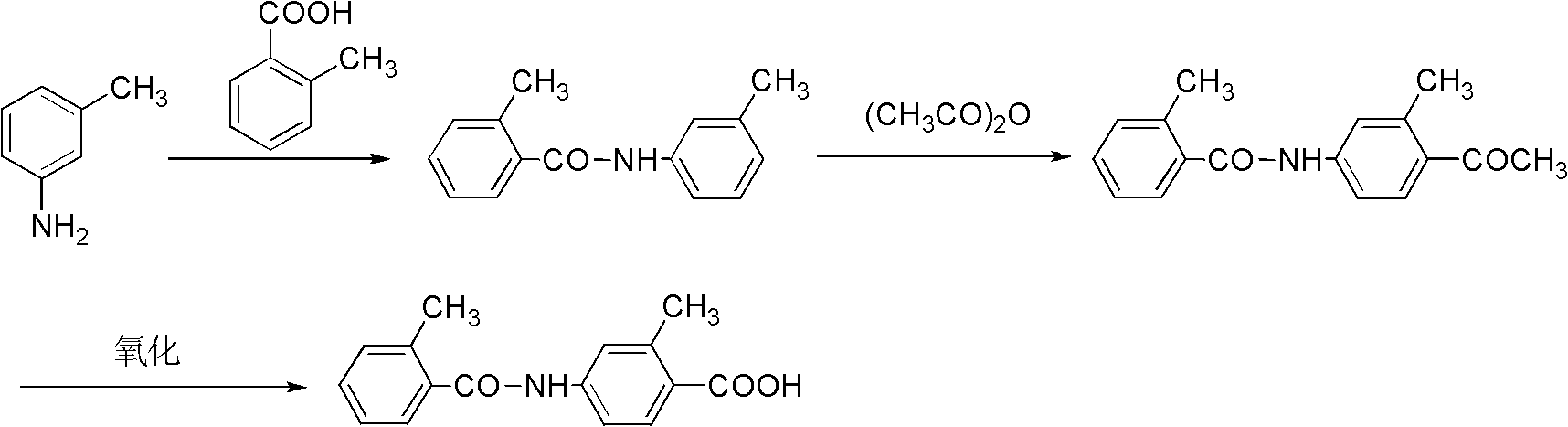
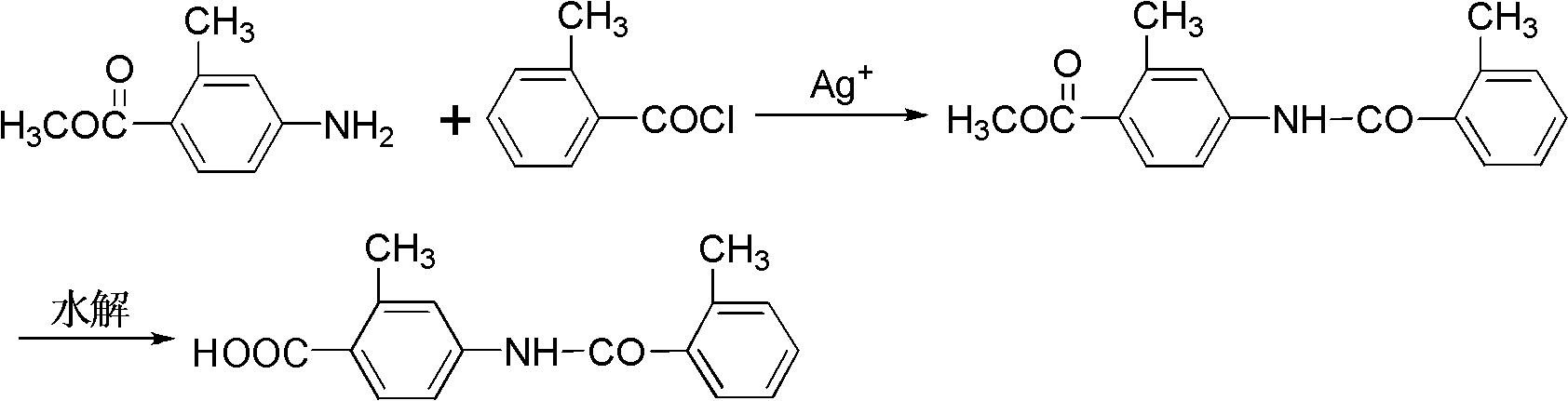
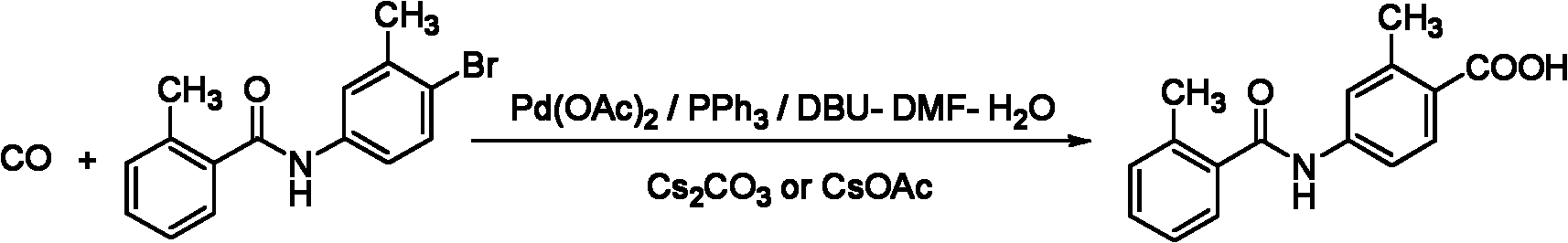Preparation method of 2-methyl-4-N-(2-methylbenzoyl)benzoic acid
A technology of methylbenzoyl and methylbenzoyl chloride, which is applied in the field of chemical pharmacy, can solve the unsatisfactory problems of safety, yield, product quality and cost control, etc., and achieves the convenience of industrial production, rapid and mild reaction , the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 2-methyl-4-amino benzoate (100g, 0.61mol) and 2-methyl-4-nitrate phenyl chloride (85g, 0.55mol) are added to 5L reactive bottle, and dichlorobial (2L 2L), Ice and salt bath cool down to -10 to 0 ° C, stir until dissolved, slowly drip and dissolve with Agno 3 (93g, 0.55 mol) a tetrahydrofucleu solution 1.5L, the drip is completed, and the reaction is 5h.After the TLC detection reaction is complete, add the saturated solution of sodium bicarbonate (500ml) to the reaction solution, stir for 10 minutes, filter, wash with a small amount of dichloromethane (600ml × 2), combine organic phase, use saturated saline water(1.5L × 3) washing, dried sodium sodium sulfate, filtering.Filtering hyperthyroidism recovery solvents obtain 2-methyl-4-n- (2-methyl benzol) benzoate (104g, 66.7 %), purity 98.9 % (HPLC, naturalization method), MS (ESI)): M / Z = 284.13 (M+H) + Essence
Embodiment 2
[0030] 2-methyl-4-amino benzoate (100g, 0.61mol) and 2-methyl-4-nitrate phenyl chloride (142g, 0.92 mol) are added to a 5L reactor, and chloroform (2L) is added.Stir at room temperature until dissolved, and slowly drip adding silver -free stem (400g, 1.83 mol) solution of 1.5L of silver (400g, 1.83 mol).After the TLC detection reaction is complete, add the saturated solution of sodium carbonate to the reaction solution (500ml), stir for 10 minutes, filter, wash with a small amount of chlorine (1L × 2), combine organic phase, use saturated salt water (1.5L of 1.5L× 3) washing, dried sodium sodium sulfate, filtering.Filtering hydraulic recovery solvents obtain 2-methyl-4-n- (2-methyl benzol) benzoate (147g, 85.1 %), purity 99.8 % (HPLC, naturalization method), MS (ESI)): M / Z = 284.13 (M+H) + Essence
Embodiment 3
[0032]2-methyl-4-amino benzoate (100g, 0.61mol) and 2-methyl-4-nitrate phenyl chloride (123g, 0.79mol) are added to 5L reactive bottle, and dichlorotide (2.5L), heat to 40 ° C, stir until dissolved, slowly drip and dissolve silver sulfonate (310g, 1.22 mol) with trifluorosaine sulfonic acid (310g, 1.22mol), 1.5L.After the TLC detection reaction is complete, add the saturated solution of sodium bicarbonate (500ml) to the reaction solution, stir for 20 minutes, filter, wash with a small amount of dichloromethane (1L × 2), combine organic phase, use saturated saline water(2L × 3) washing, dried sodium sodium sulfate, filtering.Filtering hydraulic recovery solvents obtain 2-methyl-4-n- (2-methyl benzol) benzoate (146g, 84.6 %), purity 99.3 % (HPLC, naturalization method), MS (ESI)): M / Z = 284.13 (M+H) + Essence
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com