Suspension for oral administration comprising amorphous tolvaptan
A suspension and amorphous technology, applied in the direction of medical preparations of non-active ingredients, active ingredients of heterocyclic compounds, digestive system, etc., can solve the problems of reduced solubility and absorption, and achieve excellent absorption and high solubility , the effect of delaying crystallization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
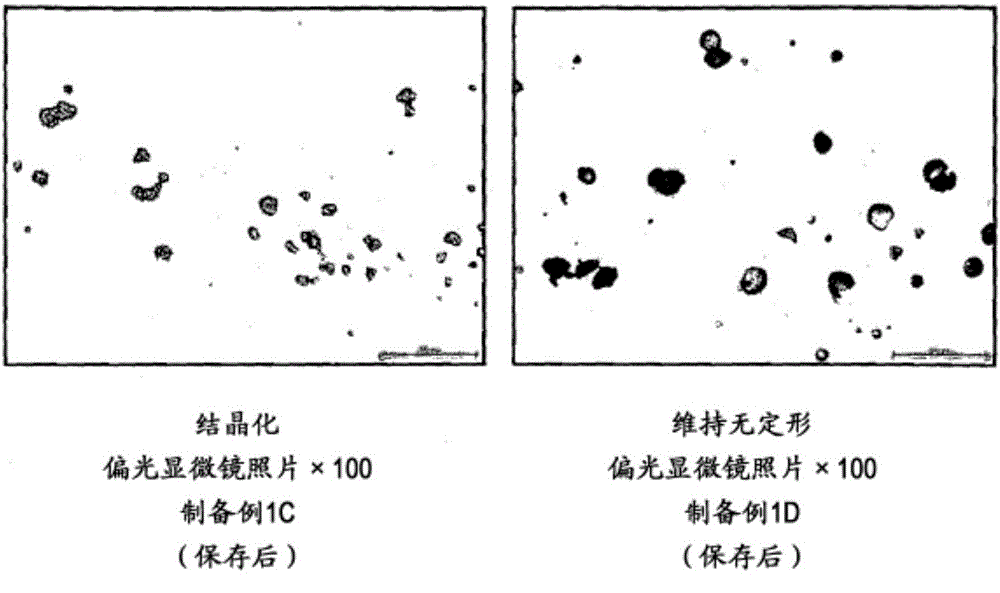
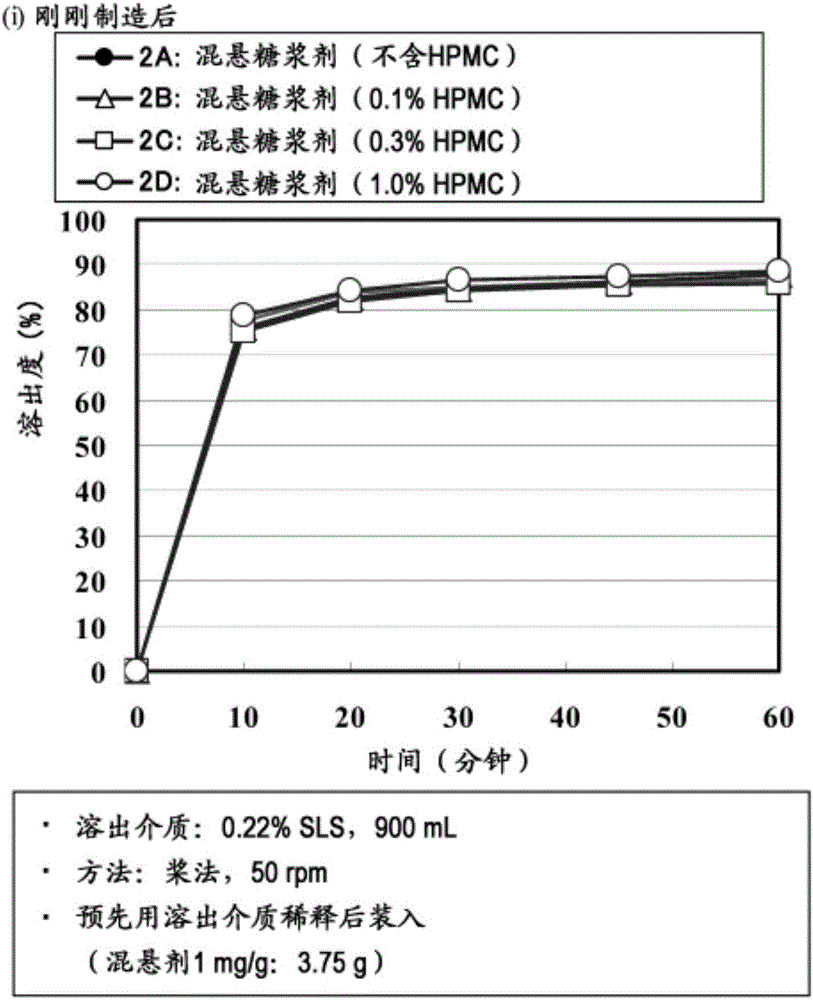
[0123] As a preferable preparation method, for example, uniformly dispersing (suspending) particles containing amorphous tolvaptan in an aqueous suspension containing hydroxypropylmethylcellulose (HPMC) or an aqueous solution containing HPMC Methods.
[0124] Specifically, the following method can be mentioned, that is, adding HPMC to purified water, and sweeteners, suspending agents, preservatives, stabilizers and / or flavoring agents added as needed to prepare an aqueous suspension or aqueous solution, and then add the particles containing amorphous tolvaptan therein to make it uniformly dispersed (suspended).
[0125] More specifically, a method comprising the steps of:
[0126] (1) Disperse the sweetener and suspending agent in purified water, stir at 79-85°C for 0.5-3 hours to obtain a uniform solution, and then cool to 27-33°C;
[0127] (2) Add preservative and stabilizer respectively and dissolve in purified water;
[0128] (3) Hydroxypropyl methylcellulose (HPMC) and...
preparation example 1A
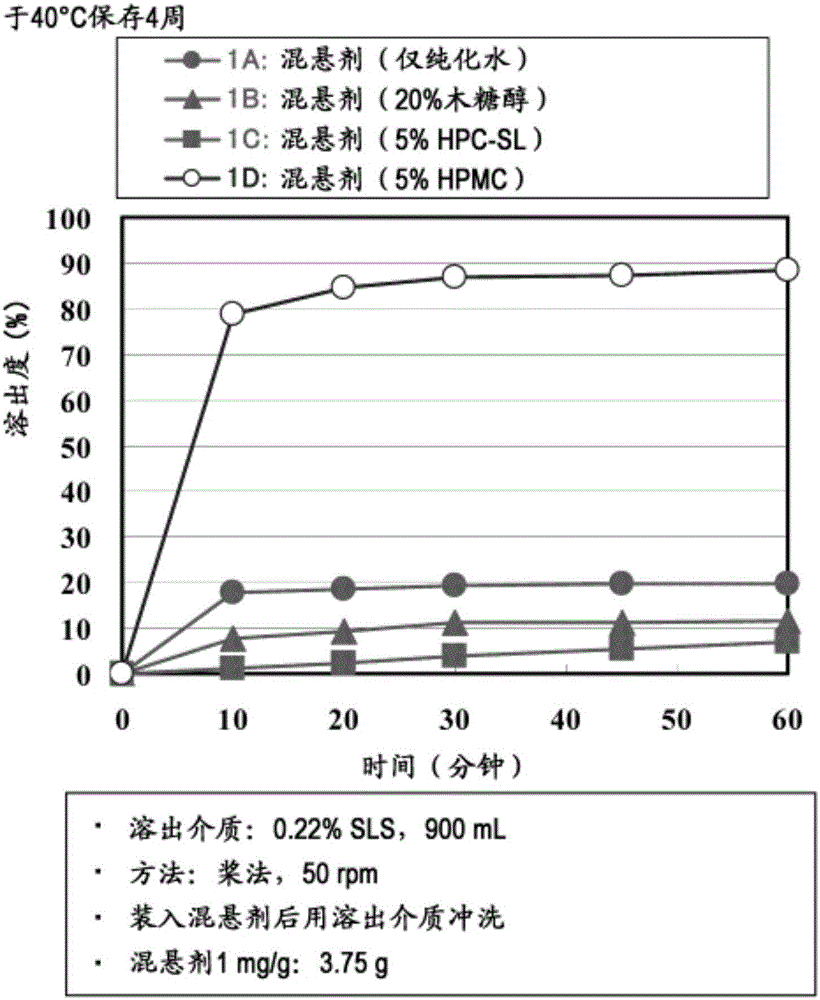
[0147] Preparation Example 1A (suspension: purified water only)
[0148] Add 0.15 g of amorphous tolvaptan-containing granules (spray-dried product; tolvaptan SD (spray-dried) powder) to 100 g of purified water, and disperse by sufficiently stirring, the amorphous tolvaptan-containing granules Tolvaptan and hydroxypropylcellulose (HPC; HPC-SL, manufactured by Nippon Soda Co., Ltd., the same below) were contained at a weight ratio of 2:1.
preparation example 1B
[0149] Preparation Example 1B (suspension: 20% xylitol)
[0150] 20 g of xylitol was added to 80 g of purified water, dissolved by sufficient stirring, and then 0.15 g of granules containing amorphous tolvaptan (spray-dried product; tolvaptan SD powder; 2:1 by weight) were added thereto. than containing tolvaptan and hydroxypropyl cellulose (HPC; HPC-SL)), dispersion is carried out by thorough stirring.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com