Suspension for oral administration containing amorphous tolvaptan
A technology of tolvaptan and suspension, which is applied in the field of suspensions for oral administration containing amorphous tolvaptan, can solve problems such as reduced solubility and absorption, and achieve excellent absorption, high solubility, The effect of retarding crystallization
Active Publication Date: 2019-05-10
OTSUKA PHARM CO LTD
View PDF12 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
When tolvaptan is prepared as a solid preparation using a common preparation technology, tolvaptan becomes crystals, and there is a problem that solubility and absorption from the gastrointestinal tract are reduced
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
preparation example Construction
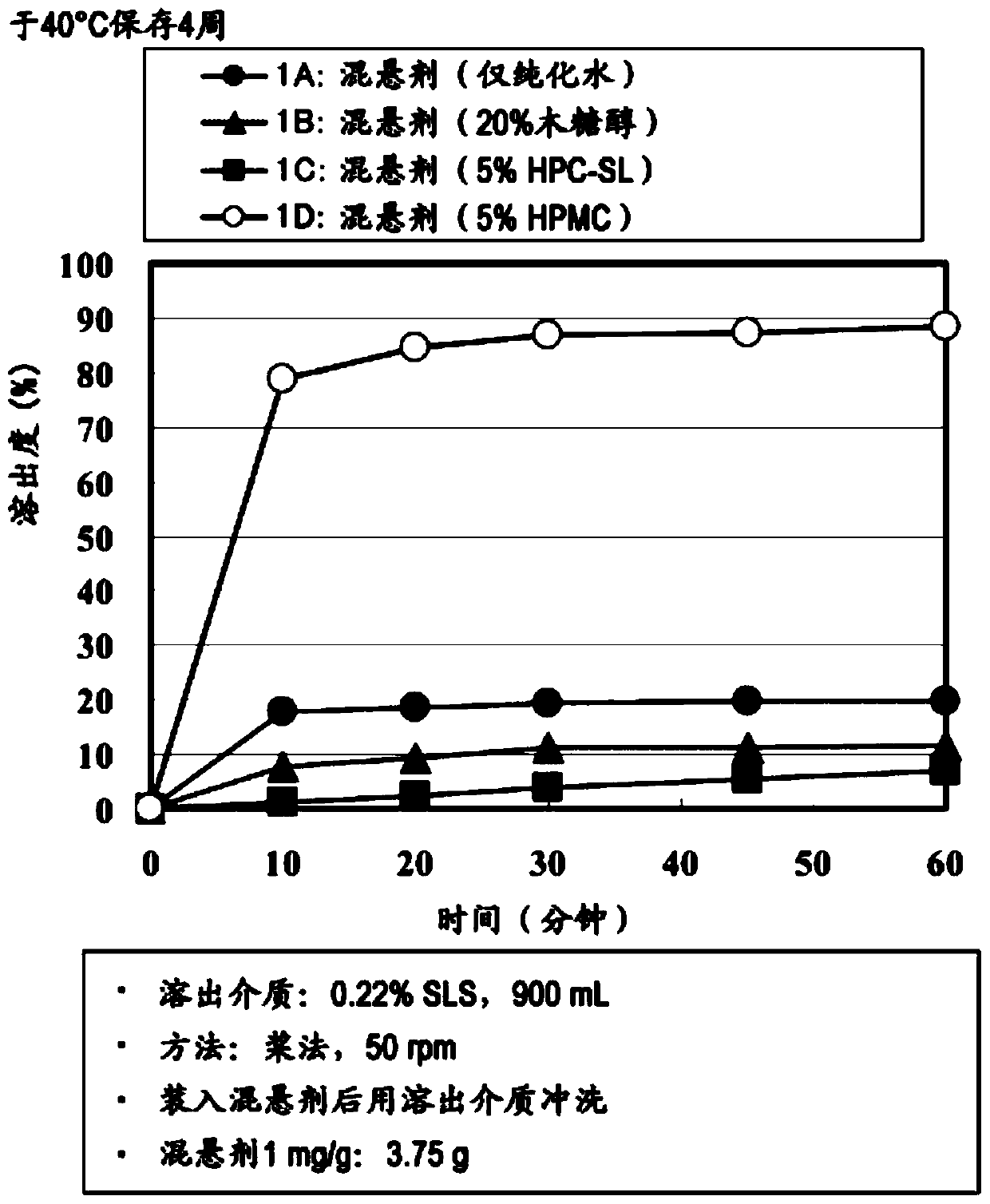
preparation example 1A
preparation example 1B
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Login to View More
Abstract
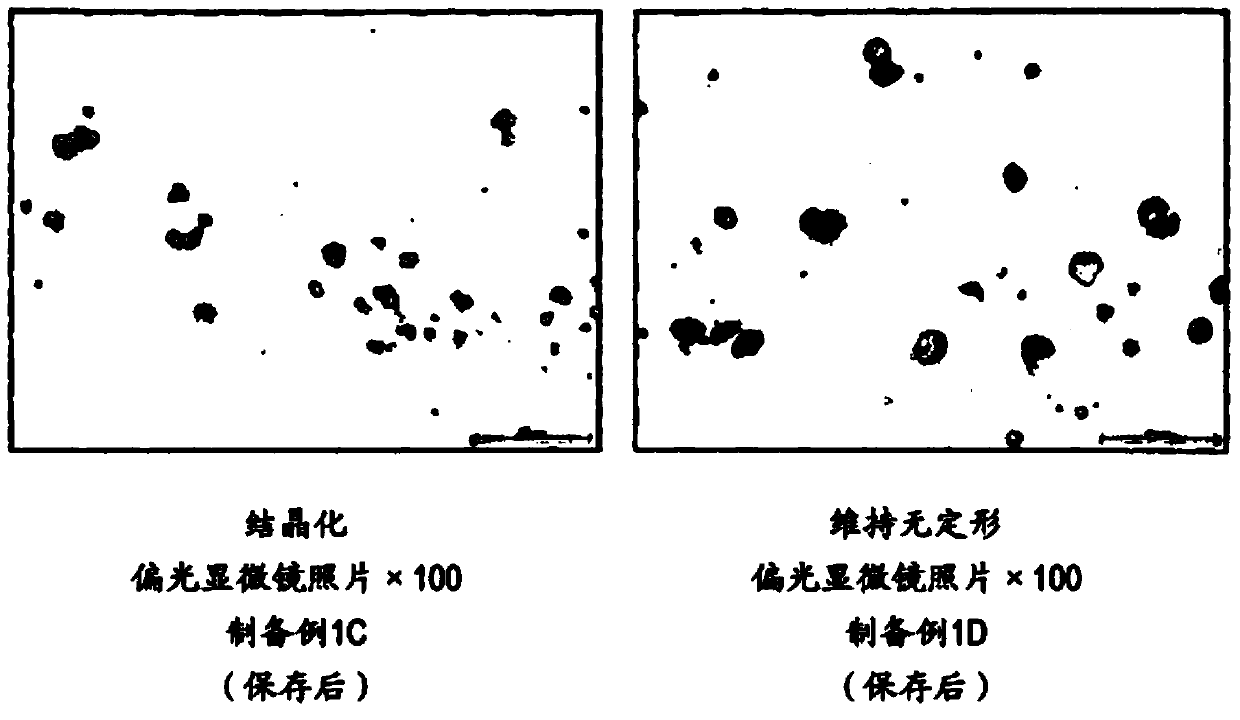
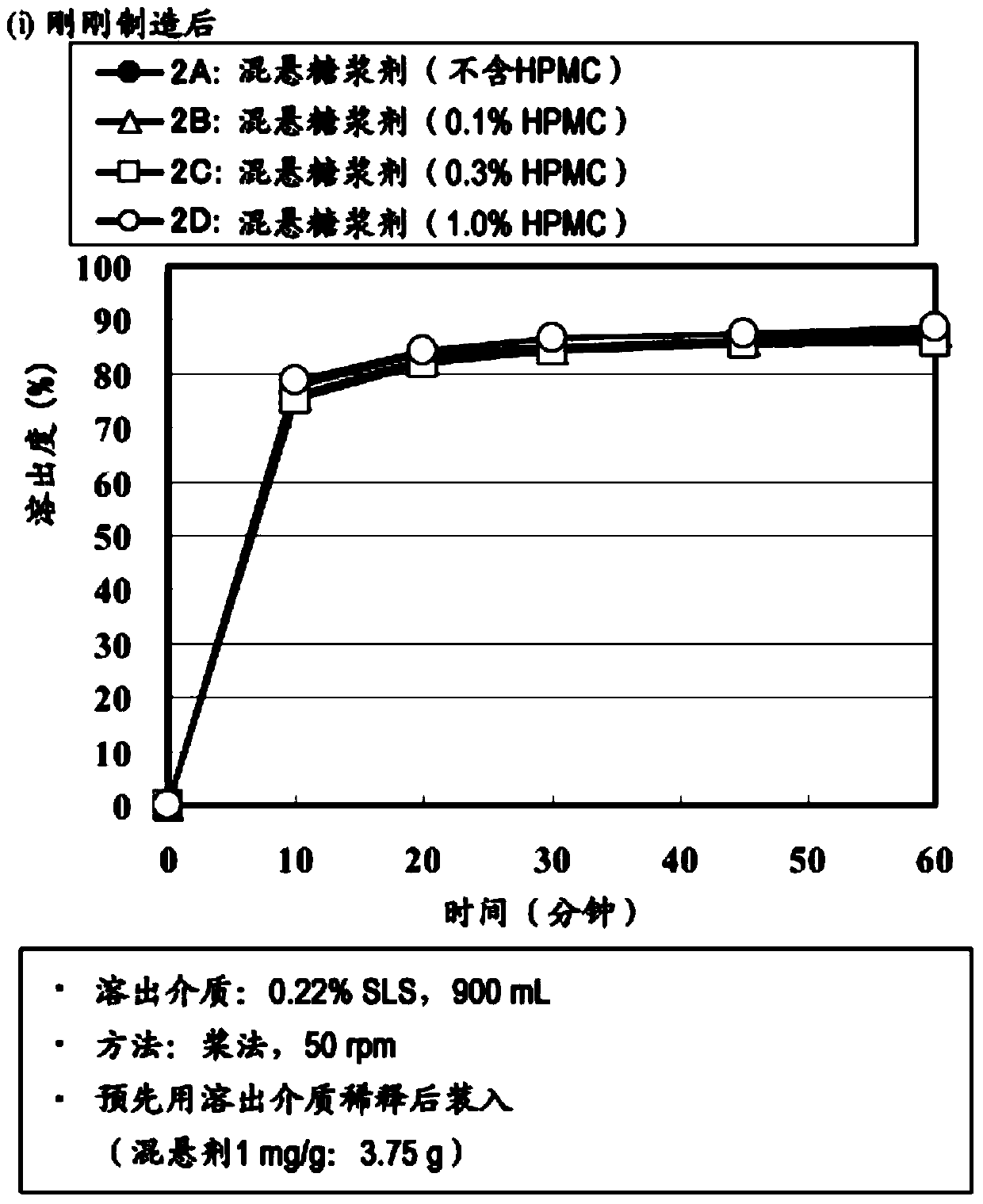
The present invention provides a suspension for oral administration, the suspension for oral administration comprises granules containing amorphous tolvaptan, capable of inhibiting or delaying the dissolution of amorphous tolvaptan in the suspension over time crystallization, and stably maintain high solubility of tolvaptan and excellent absorbability from the gastrointestinal tract; and a solid preparation for oral administration, which can be suspended when used so that Suspensions for oral administration are prepared. The present invention relates to a suspension for oral administration, especially a syrup, comprising (a) granules containing amorphous tolvaptan, (b) hydroxypropylmethylcellulose (HPMC) and (c) a solvent, wherein, Based on the total weight of the suspension for oral administration, the compounding amount of the HPMC (b) is 0.1 to 25% by weight.
Description
technical field The present invention relates to suspensions (especially syrups) for oral administration containing amorphous tolvaptan and methods for their manufacture. Background technique Tolvaptan (tolvaptan) is 7-chloro-5-hydroxyl-1-[2-methyl-4-(2-methylbenzoylamino)benzoyl]-2 shown in formula (I), 3,4,5-Tetrahydro-1H-benzazepine is a vasopressin antagonist having aquaretic activity (Patent Document 1). Tolvaptan is marketed as a therapeutic drug against hyponatremia, fluid retention in heart failure, and fluid retention in cirrhosis. When tolvaptan is prepared as a solid preparation using a common preparation technique, tolvaptan becomes a crystal, and there is a problem that the solubility and the absorbability from the gastrointestinal tract are lowered. As a means to solve the above problems, Patent Document 2 reports a means of dissolving tolvaptan and hydroxypropyl cellulose in an organic solvent, and then powdering them by spray drying to form a Compositions...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61K9/08A61K47/26A61K47/38A61K9/16A61K31/55
CPCA61K9/0095A61K47/26A61K47/38A61K9/1652A61K31/55A61P1/00A61P1/16A61P13/00A61P3/12A61P7/10A61P9/00A61P9/04A61K9/0053A61K9/10
Inventor 赤木亮之铃木海中村笃哉西林彻
Owner OTSUKA PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com