Tolvaptan solid dispersion preparation and preparation method thereof
A technology of solid dispersion and tolvaptan, which is applied in the field of tolvaptan solid dispersion preparation and its preparation, and can solve problems such as death and coma
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
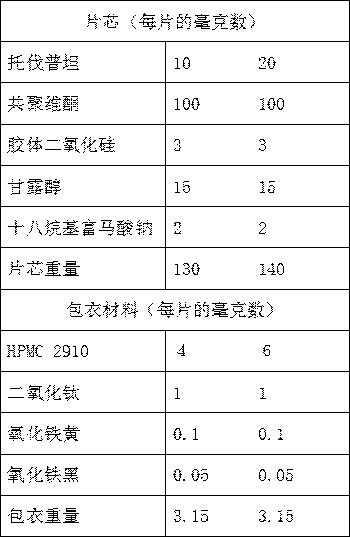
[0040] Embodiment 1: Tolvaptan solid dispersion tablet
[0041] prescription:
[0042] components
parts by weight
10
Povidone K30
100
Microcrystalline Cellulose 102
50
20
10
silica
2
Sheet weight
192
[0043] Preparation process: solvent evaporation method
[0044] Dissolve the prescribed amount of tolvaptan and povidone K30 in a solvent of acetone:methanol (1:3), volatilize under reduced pressure in a water bath at 60°C, vacuum degree 0.07-0.08MPa, and recover organic matter under reduced pressure. After the solvent becomes viscous, continue vacuum drying under reduced pressure for 1 hour, transfer to a vacuum drying oven, dry at 40°C for 48 hours, and pass through an 80-mesh sieve to pulverize to obtain a solid dispersion.
[0045] The prepared solid dispersion was added into the prescribed amount of microcrystalline cellulose pH102...
Embodiment 2
[0046] Embodiment 2: Tolvaptan solid dispersion tablet
[0047] prescription:
[0048] components
parts by weight
Tolvaptan
20
Povidone K30
100
Microcrystalline Cellulose 102
50
20
10
talcum powder
2
Sheet weight
207
[0049] Preparation process: solvent evaporation method
[0050]Take the prescribed amount of tolvaptan and povidone K30, dissolve in the solvent of methanol:dichloromethane (4:1), put in a water bath at 60°C, vacuum degree 0.07-0.08MPa, recover the organic solvent under reduced pressure, wait for After becoming viscous, continue vacuum drying under reduced pressure for 1 hour, transfer to a vacuum drying oven, dry at 40°C for 48 hours, and pass through an 80-mesh sieve to pulverize to obtain a solid dispersion.
[0051] The prepared solid dispersion is added into the prescribed amount of microcrystalline cellulose pH102, crospovi...
Embodiment 3
[0052] Embodiment 3: Tolvaptan solid dispersion tablet
[0053] prescription:
[0054] components
parts by weight
Tolvaptan
30
Povidone K30
100
Microcrystalline Cellulose 102
50
20
10
silica
2
Sheet weight
212
[0055] Preparation process: solvent evaporation method
[0056] Dissolve the prescribed amount of tolvaptan and povidone K30 in a solvent of acetone:dichloromethane (3:1), in a water bath at 55°C, with a vacuum of 0.07-0.08MPa, and recover the organic solvent under reduced pressure. After becoming viscous, continue vacuum drying under reduced pressure for 3 hours, transfer to a vacuum drying oven, dry at 60°C for 48 hours, and pass through an 80-mesh sieve to pulverize to obtain a solid dispersion.
[0057] The prepared solid dispersion is added to the prescribed amount of pregelatinized starch, low-substituted hydroxypropyl cellulose, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com