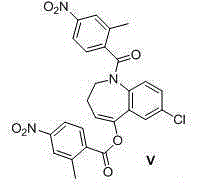
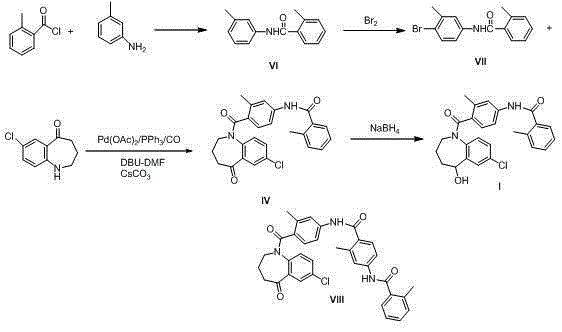
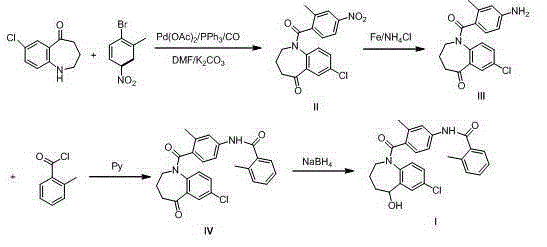
Preparation method of tolvaptan
A technology of tolvaptan and intermediates, applied in the field of preparation of tolvaptan, can solve the problems of high labor protection requirements for workers, unfavorable workshop safety operation, and large amount of solvent, so as to reduce labor protection requirements for workers and reduce purification Difficulty, improve the effect of quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Synthesis of intermediate II compound
[0028] 10.0g 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one, 11.7g 4-nitro-2-methylbromobenzene, 14.3g potassium carbonate, 3.0g Add a solution of triphenylphosphine and 0.02g palladium acetate, N,N-dimethylformamide (150ml)-water (10.0ml) into a 500ml autoclave, replace with nitrogen three times under stirring, and introduce CO gas at a pressure of 3MPa Heat at 120°C and reflux for 8 hours. Cooling, filtration to remove palladium acetate, the reaction solution to 300ml of water, stirred for 30 minutes and filtered, the solid was recrystallized with methanol and cyclohexane, and the solid was dried in vacuo at 60°C for 8 hours to obtain 17.0 g of a light yellow solid with a yield of 92.7%. HPLC purity 99.0%.
Embodiment 2
[0030] Synthesis of intermediate II compound
[0031] 10.0g 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one, 13.3g 4-nitro-2-methylbromobenzene, 14.3g potassium carbonate, 3.0g Add the solution of triphenylphosphine and 0.03g palladium acetate, N,N-dimethylformamide (150ml)-water (10.0ml) into a 500ml autoclave, replace with nitrogen three times under stirring, and introduce CO gas at a pressure of 5MPa Heat at 110°C and reflux for 8 hours. Cooling, filtering to remove palladium acetate, the reaction solution to 300ml of water, stirring for 30 minutes and filtering, the solid was recrystallized with methanol, and the solid was dried under vacuum at 60°C for 6 hours to obtain 14.0g of a light yellow solid, the yield was 76.3%, and the HPLC purity was 99.3% .
Embodiment 3
[0033] Synthesis of intermediate II compound
[0034] 10.0g 7-chloro-1,2,3,4-tetrahydrobenzo[b]azepine-5-one, 10.6g 4-nitro-2-methylchlorobenzene, 14.3g potassium carbonate, 3.0g Add a solution of triphenylphosphine and 0.04g palladium acetate, N,N-dimethylformamide (150ml)-water (10.0ml) into a 500ml autoclave, replace it with nitrogen three times under stirring, and introduce CO gas at a pressure of 3MPa Heat at 125°C and reflux for 8 hours. Cooling, filtration to remove palladium acetate, the reaction solution to such as 300ml of water, stirred for 30 minutes and filtered, the solid was recrystallized with methanol and cyclohexane, and the solid was dried in vacuo at 60°C for 8 hours to obtain 15.0 g of a light yellow solid with a yield of 81.8%. HPLC purity 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com