Tolvaptan solid preparation
A technology for tolvaptan and solid preparation, which is applied in the field of solid preparations containing tolvaptan, can solve the problems of unfavorable product promotion, complicated hydroxypropyl beta cyclodextrin process and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Component
[0045] The preparation process of tolvaptan tablets: add povidone and tolvaptan into 400ml absolute ethanol and heat until the solution is clear, weigh the lactose, part of pregelatinized starch, and microcrystalline cellulose into the Glatt flow The fluidized bed adopts a one-step granulation process for granulation. After the granulation is completed, the water content is measured and the granulation is passed through a 30-mesh sieve. After adding low-substituted hydroxypropyl cellulose and mixing uniformly, add magnesium stearate, mix for 5 minutes and then press tablets.
Embodiment 2
[0047] Component
[0048] The preparation process of the composition of tolvaptan and copovidone: adding copovidone and tolvaptan into 750ml of acetone and stirring until the solution is clear, and spray drying to obtain the composition of tolvaptan and copovidone.
[0049] Component
[0050] Preparation of tolvaptan tablets: Weigh the prescribed amount of tolvaptan composition, lactose, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose, and mix them uniformly by an equal addition method, then add magnesium stearate and mix for 5 minutes After pressing the tablet.
Embodiment 3
[0052] Component
[0053] Tolvaptan and povidone composition preparation process: Povidone and tolvaptan are added to a mixed solvent of 180ml absolute ethanol and 520ml dichloromethane, after dissolving, tolvaptan is obtained by drying under reduced pressure combination.
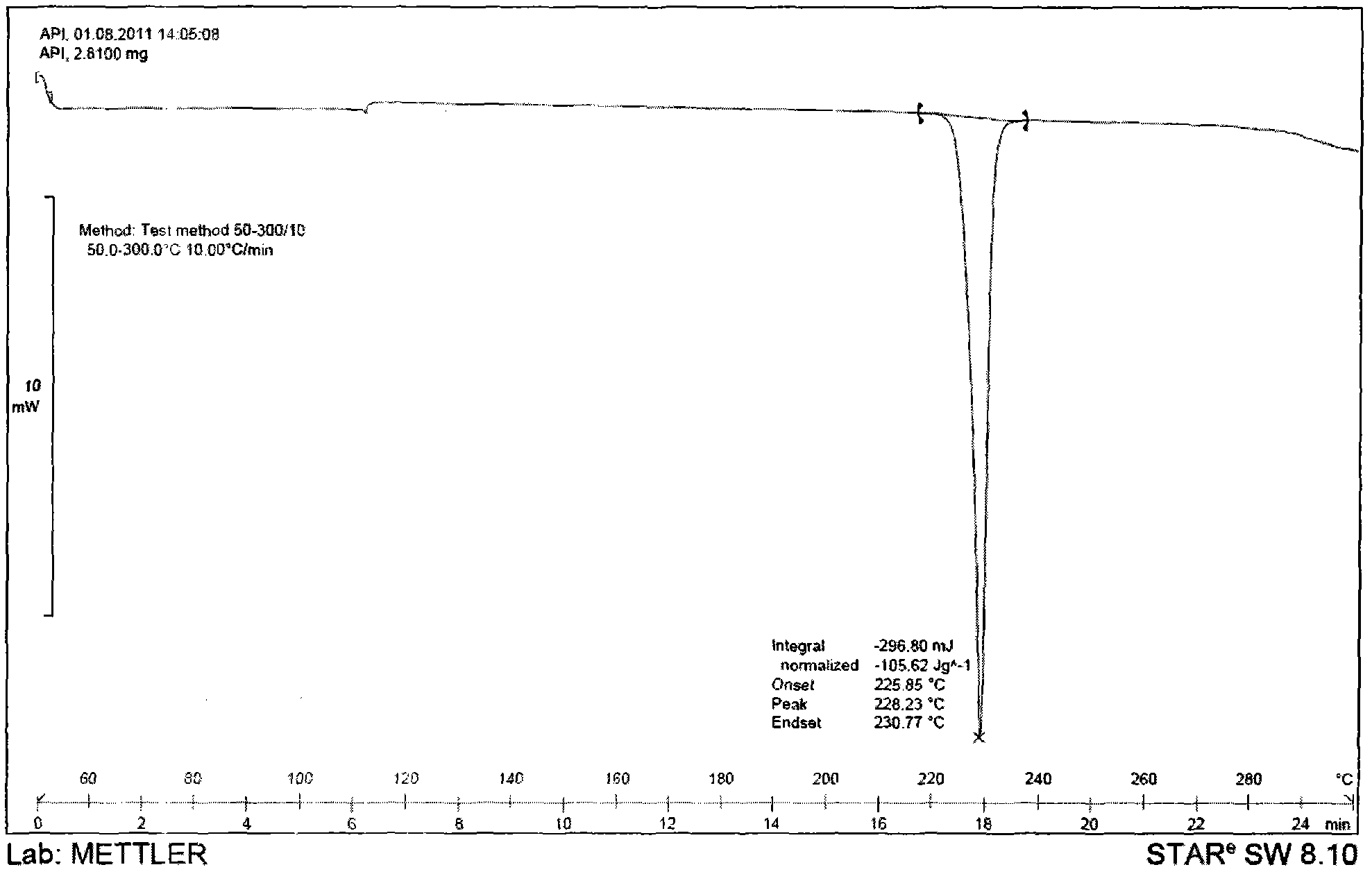
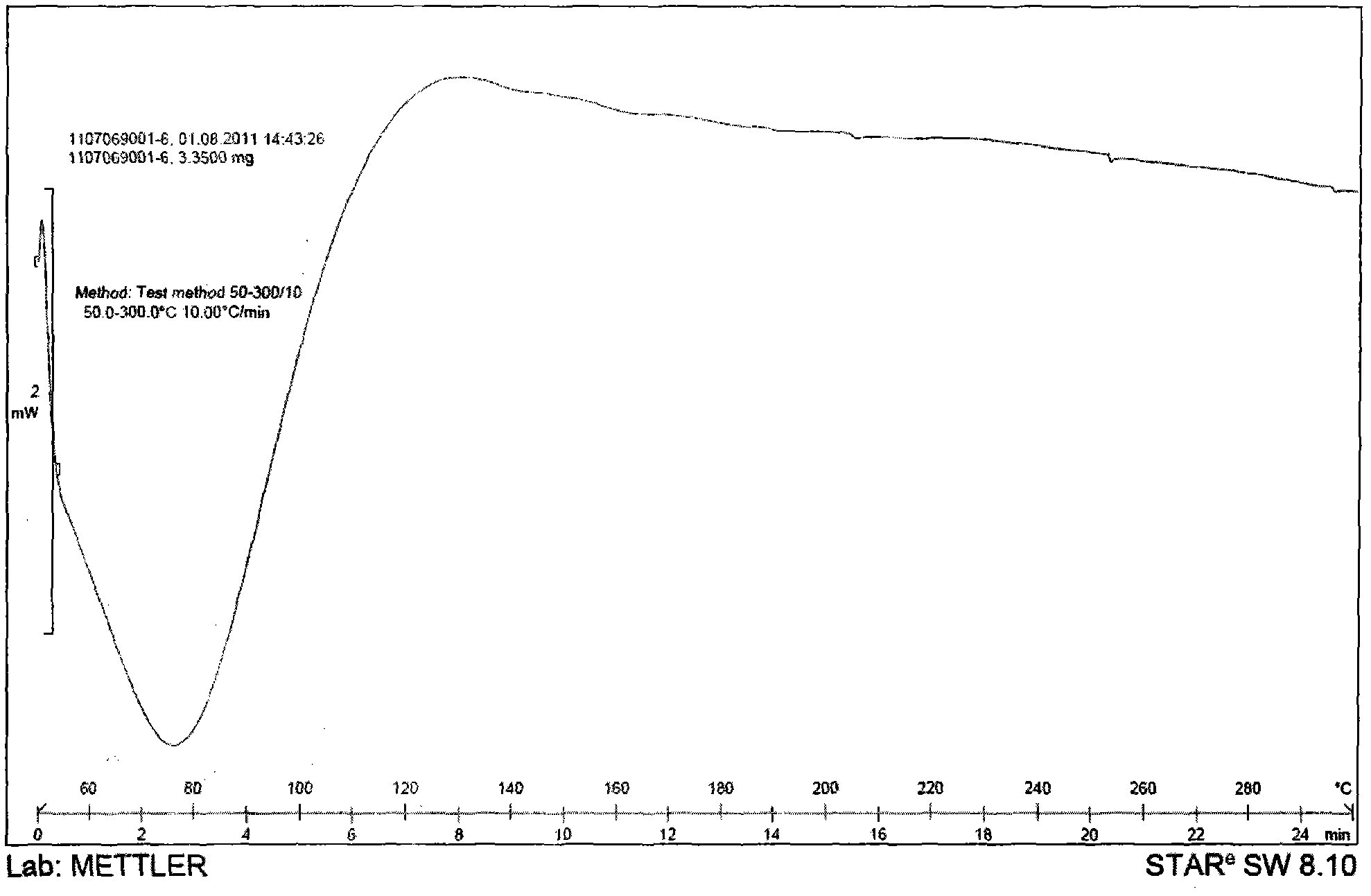
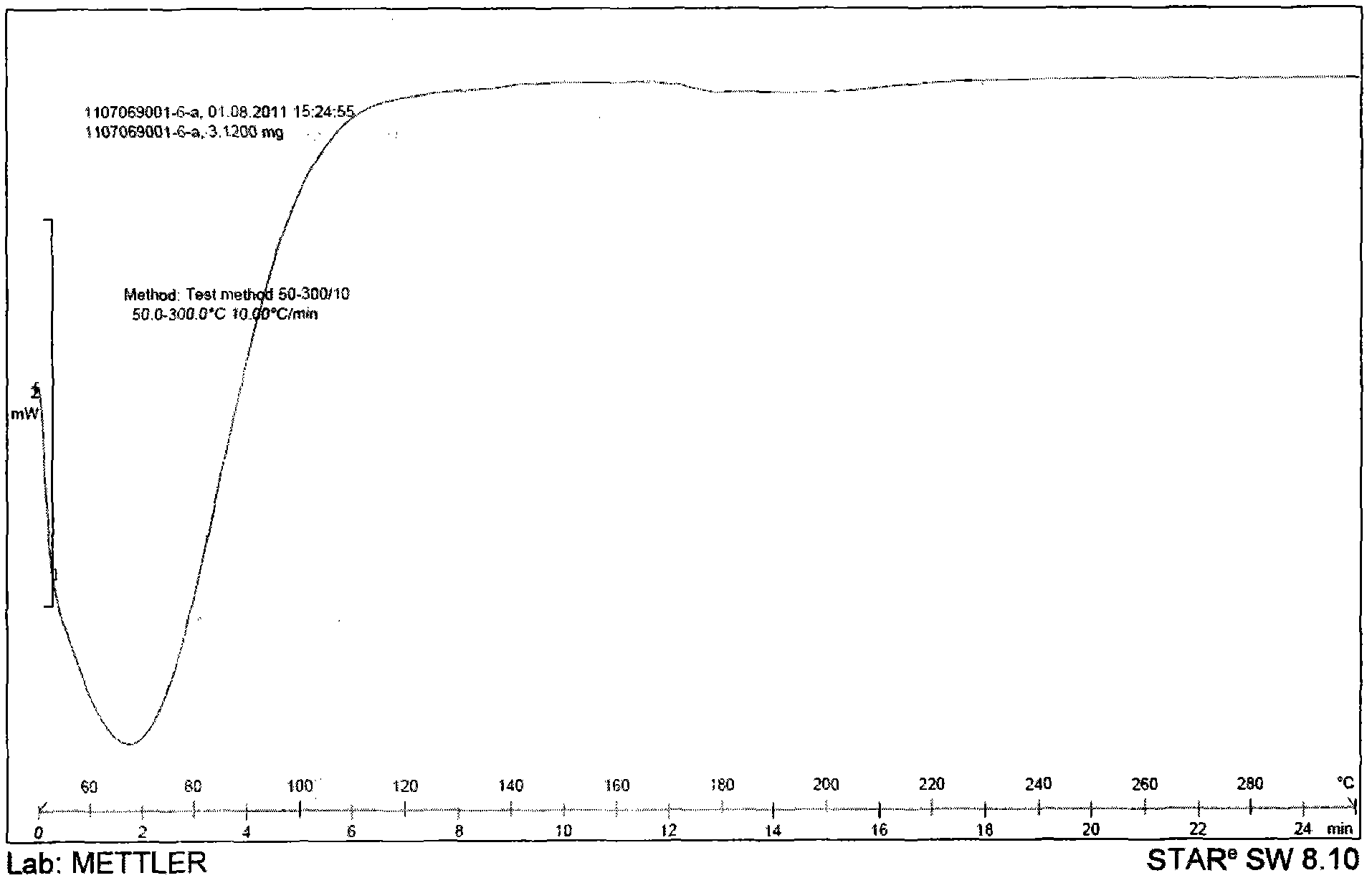
[0054] Weigh an appropriate amount of the povidone, tolvaptan, and the obtained combination of tolvaptan and povidone for the above prescription, and use differential calorimetry (DSC) measurement and X-powder diffraction (XRPD) Measure, get attached Figure 1-6 The results showed that the tolvaptan and povidone composition obtained were amorphous in tolvaptan.
[0055] Component
[0056] The preparation process of tolvaptan tablets: Weigh the prescribed amount of tolvaptan composition, lactose, partially pregelatinized starch, microcrystalline cellulose, and cross-linked povidone, and mix them evenly by equal addition, then add Magnesium stearate, mixed for 5 minutes and then compressed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com