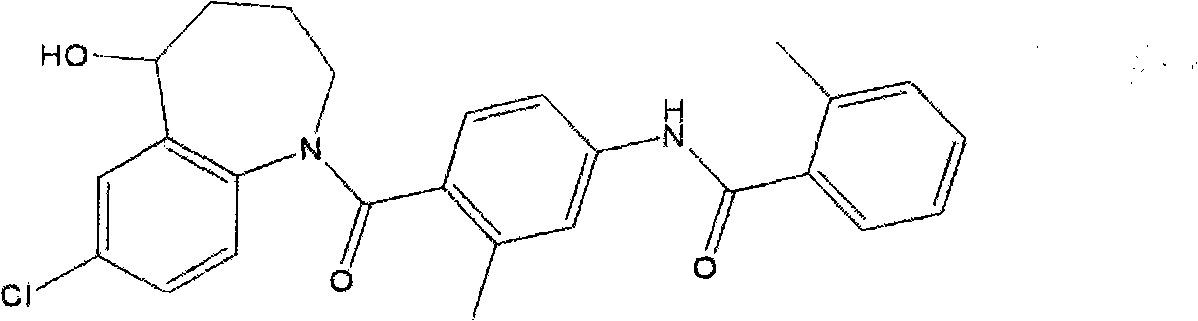
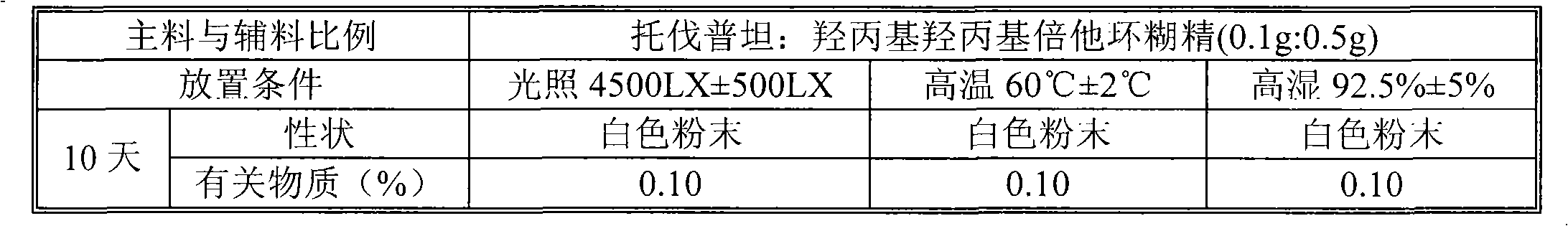
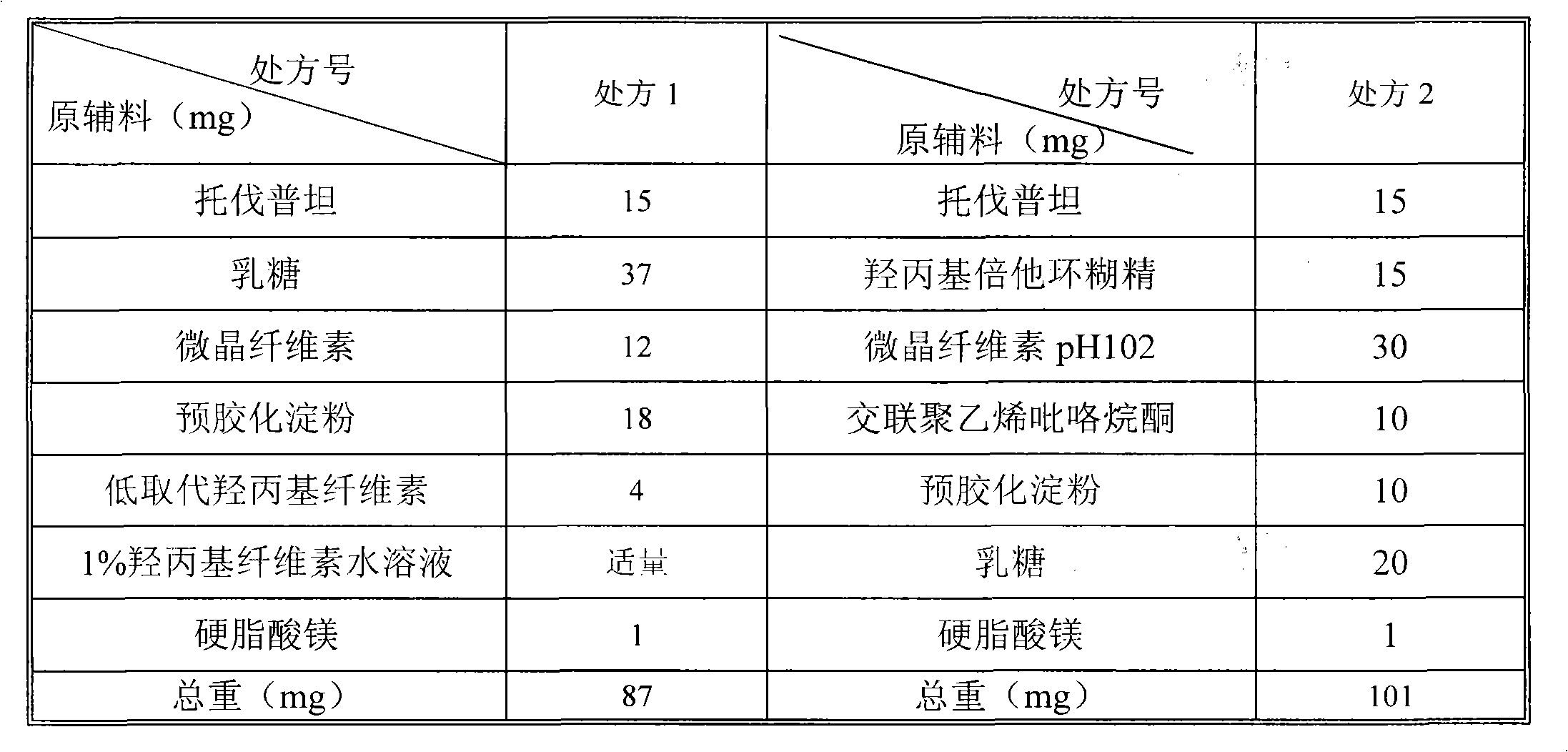
Tolvaptan medicinal composition and preparation method thereof
A technology of tolvaptan and a composition, applied in the field of medicine, can solve the problems of insufficient absorption in the gastrointestinal tract, limited dosage form and route of administration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The recipe for making 1000 tablets is as follows:
[0045] Raw materials
Weight (G)
Tolvaptan
15
Hydroxypropyl Beta Cyclodextrin
15
Microcrystalline Cellulose pH102
30
Cross-linked polyvinylpyrrolidone
10
10
20
1
[0046] steps include
[0047] a) Preparation and processing of raw and auxiliary materials: the raw materials are processed by jet milling; they are mixed with the prescribed amount of hydroxypropyl beta-cyclodextrin, ground and pulverized to make a mixture powder.
[0048] b) Weighing and mixing: Weigh the above-mentioned mixture and other auxiliary materials according to the prescription amount and calculate the feeding amount after checking by two people, and mix them evenly in an equal and incremental manner.
[0049] c) Intermediate inspection: Take the above-mentioned uniformly mixed raw and auxiliary ...
Embodiment 2
[0054] The recipe for making 1000 tablets is as follows:
[0055] Raw materials
Weight (G)
Tolvaptan
30
Hydroxypropyl Beta Cyclodextrin
30
Microcrystalline Cellulose pH102
60
Cross-linked polyvinylpyrrolidone
20
20
40
2
[0057] steps include
[0058] a) Preparation and processing of raw and auxiliary materials: the raw materials are processed by jet milling; they are mixed with the prescribed amount of hydroxypropyl beta-cyclodextrin, ground and pulverized to make a mixture powder.
[0059] b) Weighing and mixing: Weigh the above-mentioned mixture and other auxiliary materials according to the prescription amount and calculate the feeding amount after checking by two people, and mix them evenly in an equal and incremental manner.
[0060] c) Intermediate inspection: Take the above-mentioned uniformly mixed...
Embodiment 3
[0065] The recipe for making 1000 tablets is as follows:
[0066] Raw materials
Weight (MG)
Tolvaptan
60
Hydroxypropyl Beta Cyclodextrin
60
Microcrystalline Cellulose pH102
120
Cross-linked polyvinylpyrrolidone
40
pregelatinized starch
40
lactose
80
Magnesium stearate
4
[0067] steps include
[0068] a) Preparation and processing of raw and auxiliary materials: the raw materials are processed by jet milling; they are mixed with the prescribed amount of hydroxypropyl beta-cyclodextrin, ground and pulverized to make a mixture powder.
[0069] b) Weighing and mixing: Weigh the above-mentioned mixture and other auxiliary materials according to the prescription amount and calculate the feeding amount after checking by two people, and mix them evenly in an equal and incremental manner.
[0070] c) Intermediate inspection: Take the above-mentioned uniformly mixed raw and a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com