Tolvaptan crystal and medicine composition thereof
A technology for tolvaptan and composition, which is applied in the field of organic drug synthesis and can solve the problems of requiring higher temperature, difficulty in large production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
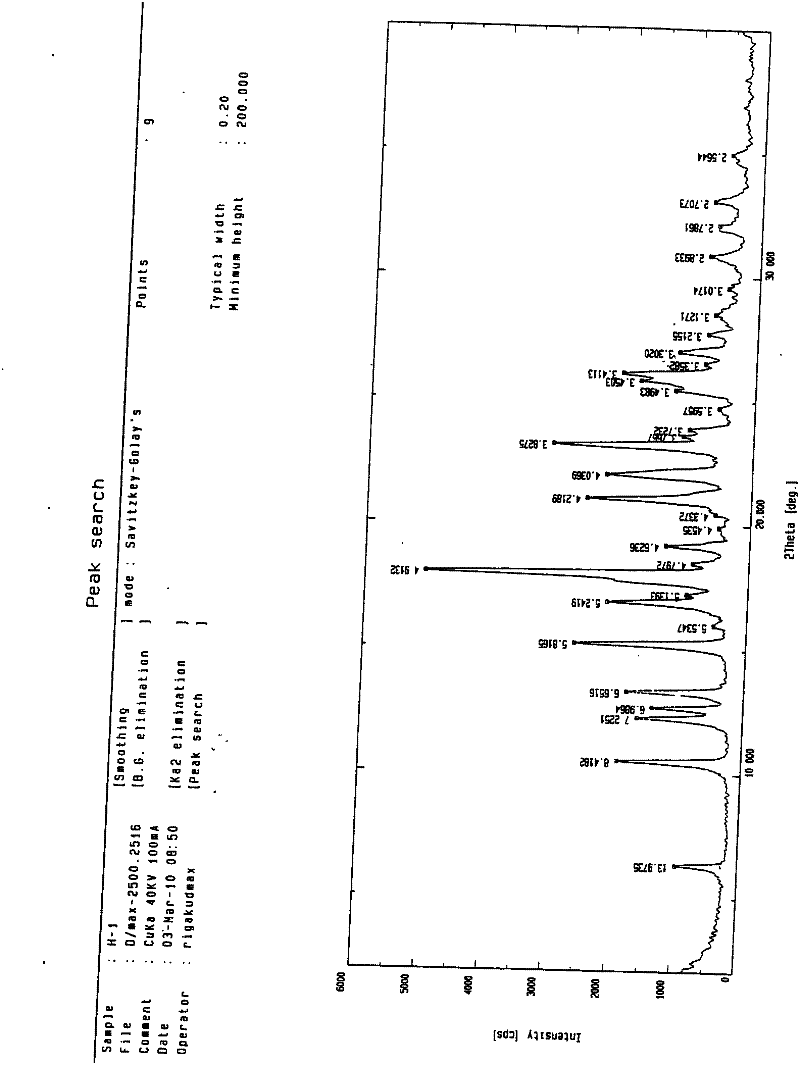
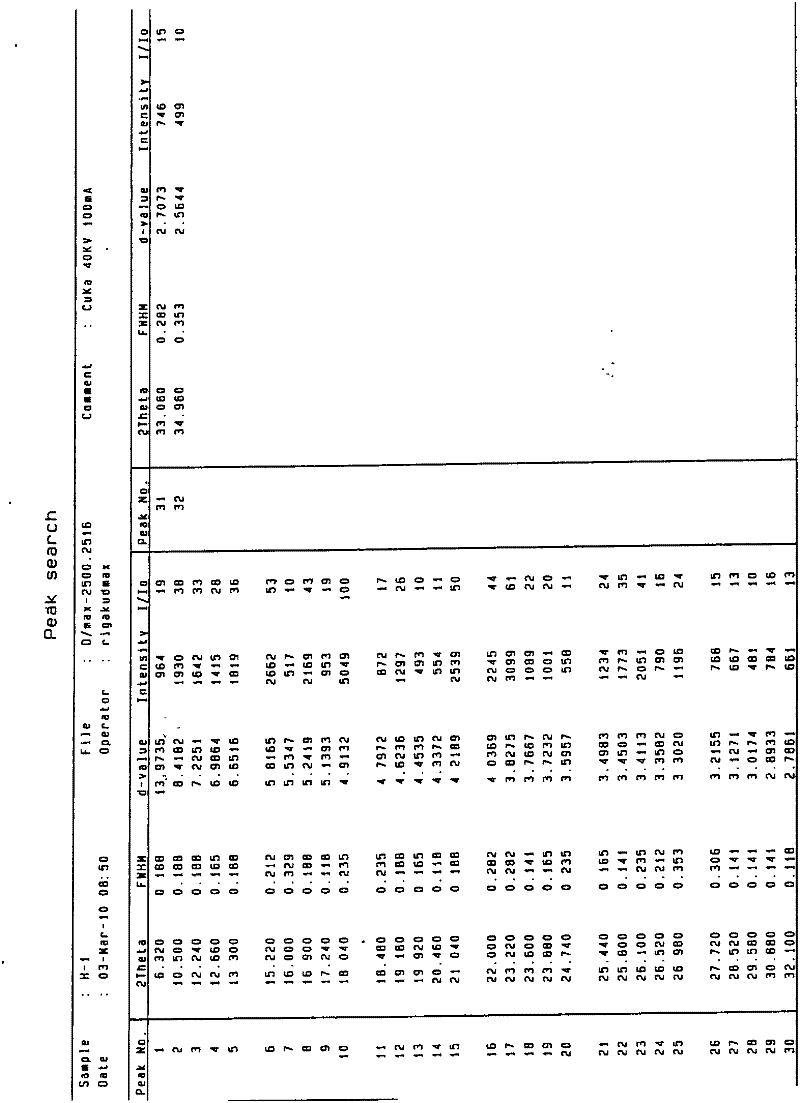
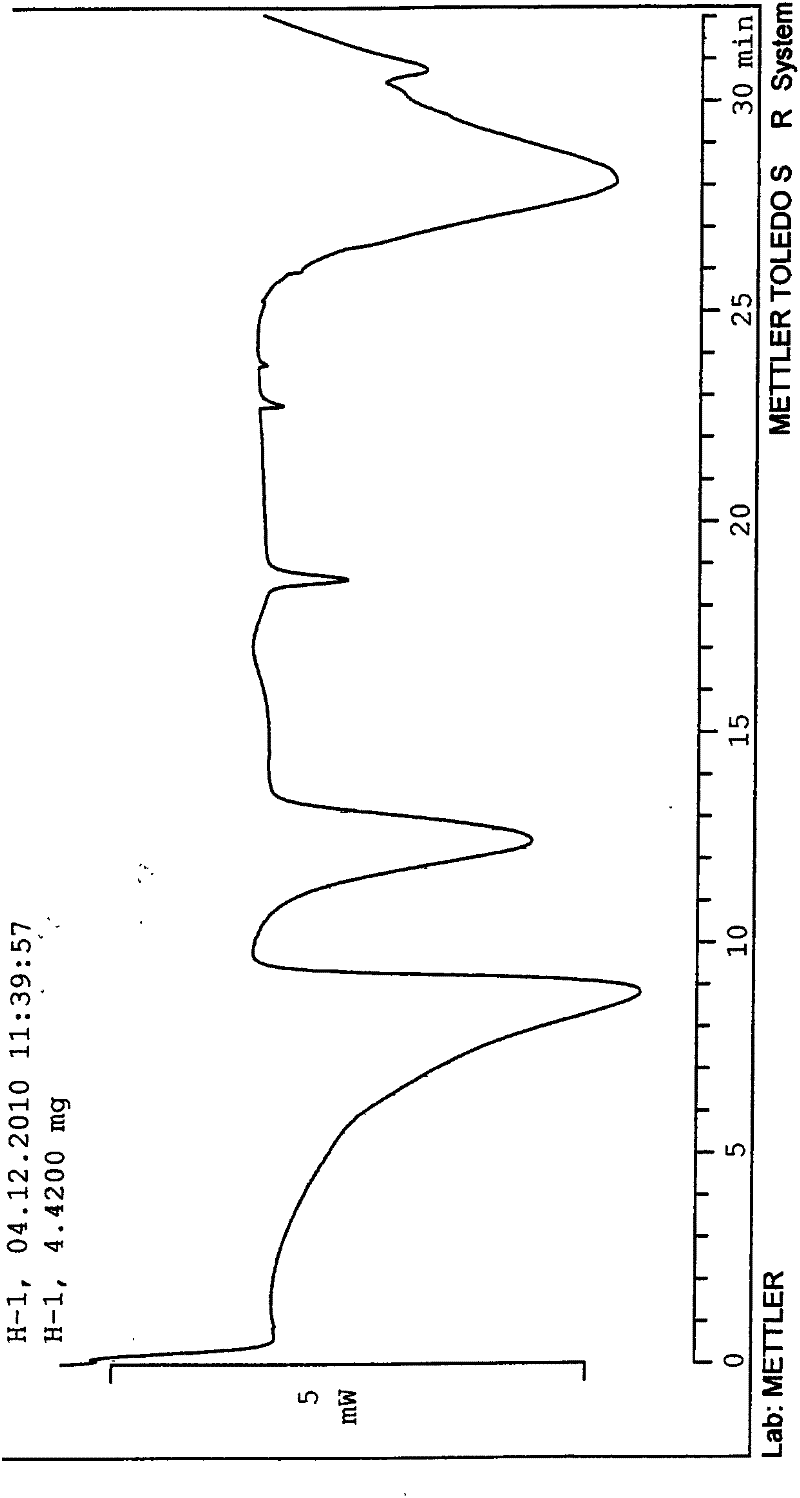
[0100] Heat and dissolve 1180 g of the crude product with 20 times of acetone (23.6 L), then lower it to 25° C., add dropwise 10 times of pure water (11.8 L) under stirring, and precipitate a large amount of solid after stirring for 2 hours, keep stirring at 30° C. for 6 hours, and then Cool down to 0°C and stir for 4 hours, filter, and vacuum-dry at 50°C to a constant weight of 1120g, yield 94.9%, HPLC 99.81%. 0.5 crystalline water tolvaptan crystals were obtained (see Figure 1 for the X-ray powder diffraction pattern), with a melting point of 155-156.7°C.
Embodiment 2
[0102] Heat and dissolve 20 g of the crude product with 30 times of ethanol (600ml), then lower it to 30°C, add 15 times of pure water (300ml) dropwise under stirring, and precipitate a large amount of solid after stirring for 2 hours, keep stirring at 25°C for 3 hours, and then lower the temperature for 2 hours. Stir at ℃ for 4 hours, filter, and vacuum-dry at 50℃ to a constant weight of 18g, yield 90%, HPLC 99.6%. 0.5 crystalline water tolvaptan crystals were obtained (see Figure 1 for the X-ray powder diffraction pattern), with a melting point of 155-156° C. (content 99.81%).
Embodiment 3
[0104] Heat and dissolve 20g of the crude product in 18 times ethanol (360ml), then lower it to 25°C, add 9 times the amount of pure water (180ml) dropwise under stirring, and after stirring for 3 hours, a large amount of solid precipitates, keep stirring at 28°C for 4 hours, and then lower the temperature by 1°C Stir for 4 hours, filter, and vacuum-dry at 50°C to a constant weight of 16g, yield 80%, HPLC 99.9%. 0.5 crystalline water tolvaptan crystals were obtained (see Figure 1 for the X-ray powder diffraction pattern), with a melting point of 155.2-156° C. (content 99.81%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com