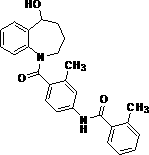
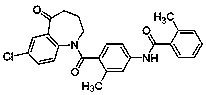
Preparation method of high-purity tolvaptan
A tolvaptan, high-purity technology, applied in the field of drug preparation, can solve the problems of dechlorination impurity residue, inability to completely inhibit dechlorination reaction, and inability to obtain high-purity tolvaptan, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1)
[0031] The preparation method of the high-purity tolvaptan of the present embodiment is as follows:
[0032] Add 5g of the compound of formula II into a 100mL three-neck flask, then add 25mL of tetrahydrofuran, stir to dissolve, cool down to -5~0°C, control the temperature at -5~0°C, and dropwise add 1.73g of dihydrofuran with a concentration of 70wt%. Hydrogen bis(2-methoxyethoxy)sodium aluminate toluene solution, heat up to 10-20°C after addition, and stir for 2 hours.
[0033] After the completion of the reaction monitored by HPLC, the temperature was controlled at 10-20°C, and 50mL of water was added dropwise. Solids were precipitated. Stirring was continued for 1-2h, and suction filtration was performed. The filter cake was recrystallized with methanol aqueous solution, and dried under reduced pressure to obtain white crystals of tolvaptan. 4.73g, the yield was 94.2%, the HPLC purity was 99.97%, and the dechlorinated impurity IV was not detected.
Embodiment 2~ Embodiment 6)
[0035] The preparation method of each embodiment is basically the same as that of Example 1, and the differences are shown in Table 1.
[0036] Table 1
[0037]
Embodiment 7~ Embodiment 10)
[0039] The preparation method of each embodiment is basically the same as that of Example 1, and the differences are shown in Table 2.
[0040] Table 2
[0041]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com