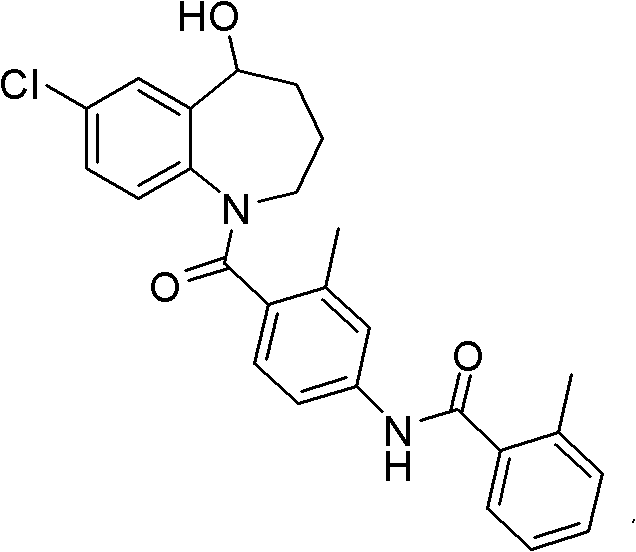
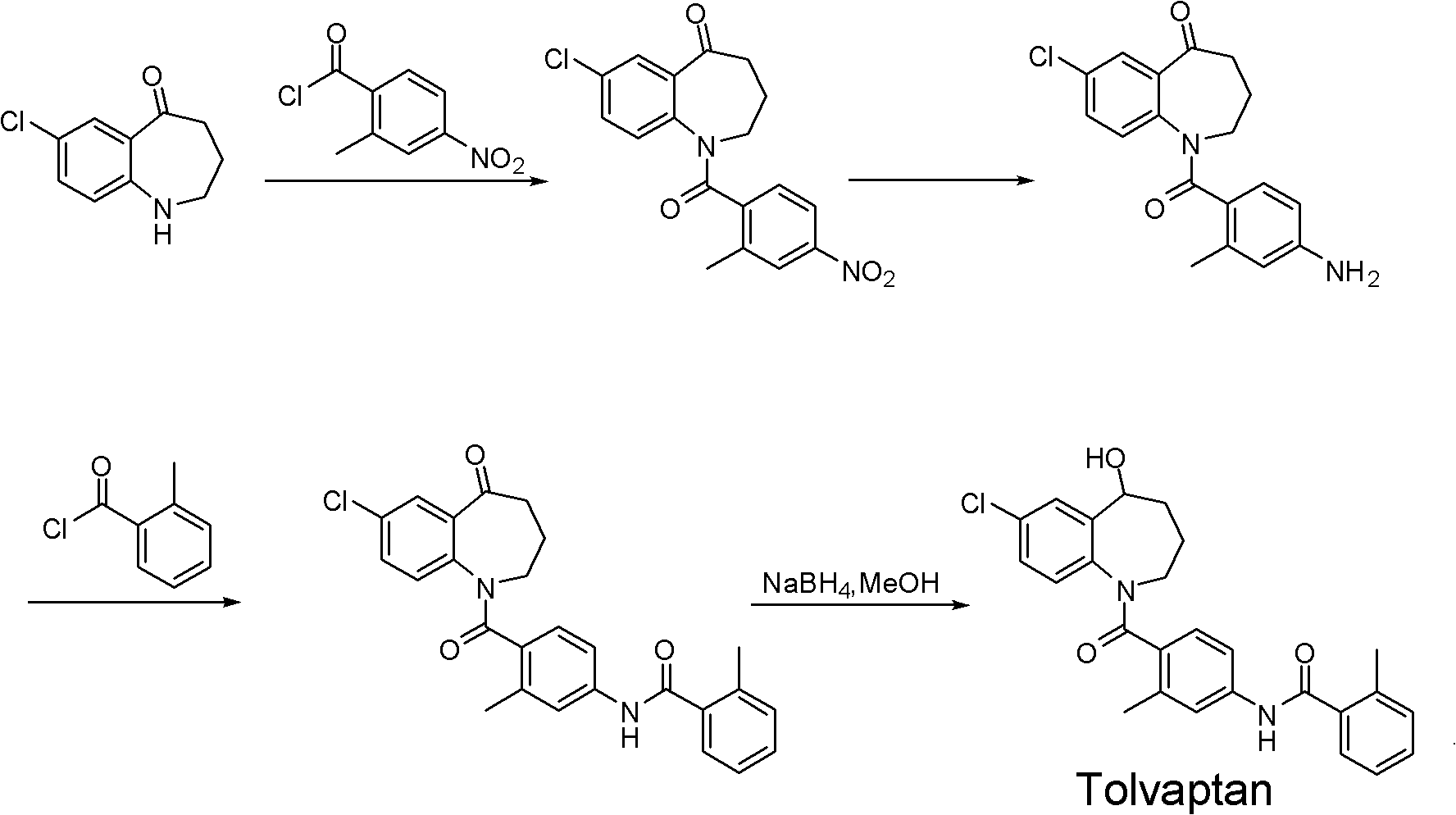
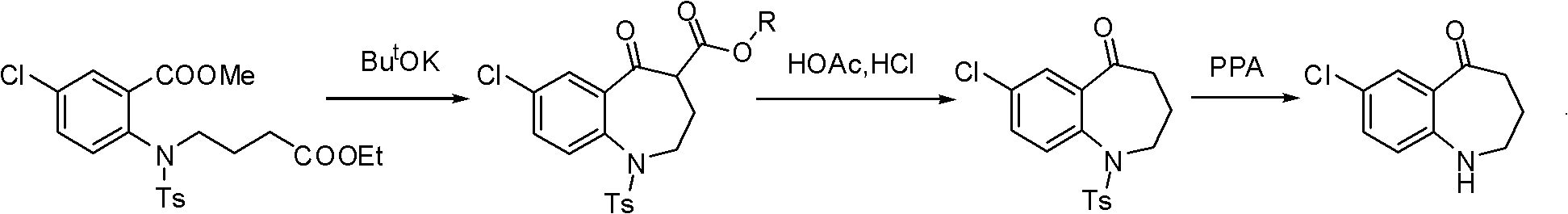
Tolvaptan intermediate and preparation method thereof
A compound, benzyl technology, applied in the field of tolvaptan intermediates and its preparation, can solve the problems of large-scale production material transfer and difficult reaction operation, high viscosity of polyphosphoric acid, low reaction yield, etc., to achieve simple operation, Environmental friendliness and the effect of improving the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine-4-carboxylic acid methyl ester
[0050] In a 500mL four-neck round bottom flask equipped with a magnetic stirrer and a thermometer, add 7-chloro-5-oxo-1-p-toluenesulfonyl-2,3,4,5-tetrahydro-1H-1-benzene 8.1 g of methyl azepine-4-carboxylate, 2.0 g of concentrated sulfuric acid. React at 40°C for 2 hours, TLC shows that the reaction is complete, then cool with an ice-salt bath, slowly add saturated sodium bicarbonate solution dropwise, adjust the pH to 7-8, extract with 100mL ethyl acetate, wash the organic phase with brine after liquid separation, anhydrous magnesium sulfate After drying, filtering and evaporating the solvent, silica gel column chromatography obtained 4.4 g of the compound represented by formula I as a light yellow oily liquid with a yield of 87.6%.
[0051] ESI-MS(m / z): 254(M+H), 277(M+Na), 293(M+K)
[0052] 1 HNMR: (400MHz, CDCl 3 )2.10(2H, m), 3.16(2H, m), 3.77(1H, t), 3...
Embodiment 2
[0053] Example 2: Preparation of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine-4-carboxylic acid ethyl ester
[0054]In a 500mL four-neck round bottom flask equipped with a magnetic stirrer and a thermometer, add 7-chloro-5-oxo-1-p-toluenesulfonyl-2,3,4,5-tetrahydro-1H-1-benzene 8.4 g of ethyl azepine-4-carboxylate, 122.5 g of 80% phosphoric acid. React at 100°C for 2 hours, TLC shows that the reaction is complete, cool with ice-salt bath, slowly add saturated sodium bicarbonate solution dropwise, adjust the pH to 7-8, extract with 100mL ethyl acetate, wash the organic phase with brine after liquid separation, and anhydrous magnesium sulfate After drying, filtering and evaporating the solvent, silica gel column chromatography obtained 4.8 g of the compound represented by formula I as a pale yellow oily liquid with a yield of 89.1%.
[0055] ESI-MS(m / z): 268(M+H), 290(M+Na), 306(M+K)
[0056] 1 HNMR: (400MHz, CDCl 3 )1.30(3H, t), 2.15(2H, m), 3.11(2H, m), 3.85(1H, t), ...
Embodiment 3
[0057] Example 3: Preparation of 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine-4-carboxylate
[0058] In a 500mL four-neck round bottom flask equipped with a magnetic stirrer and a thermometer, add 7-chloro-5-oxo-1-p-toluenesulfonyl-2,3,4,5-tetrahydro-1H-1-benzene 8.1 g of methyl azepine-4-carboxylate, 20.0 g of concentrated sulfuric acid, and 33.8 g of polyphosphoric acid. React at 70°C for 2 hours, TLC shows that the reaction is complete, then cool with an ice-salt bath, slowly add saturated sodium bicarbonate solution dropwise, adjust the pH to 7-8, extract with 100mL ethyl acetate, wash the organic phase with brine after liquid separation, anhydrous magnesium sulfate After drying, filtering and evaporating the solvent, silica gel column chromatography obtained 4.2 g of the compound represented by formula I as a light yellow oily liquid with a yield of 83.6%.
[0059] ESI-MS(m / z): 254(M+H), 277(M+Na), 293(M+K)
[0060] 1 HNMR: (400MHz, CDCl 3 )2.10(2H, m), 3.16(2H,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com