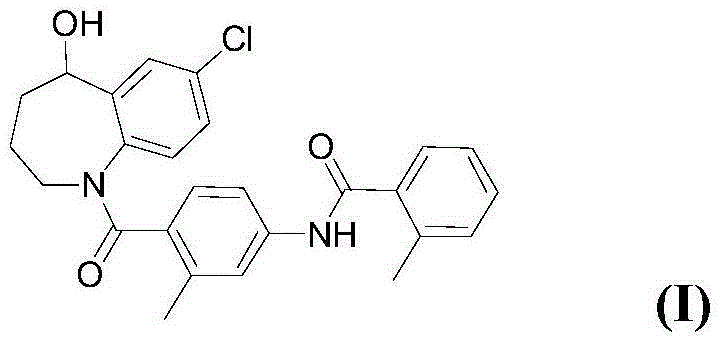
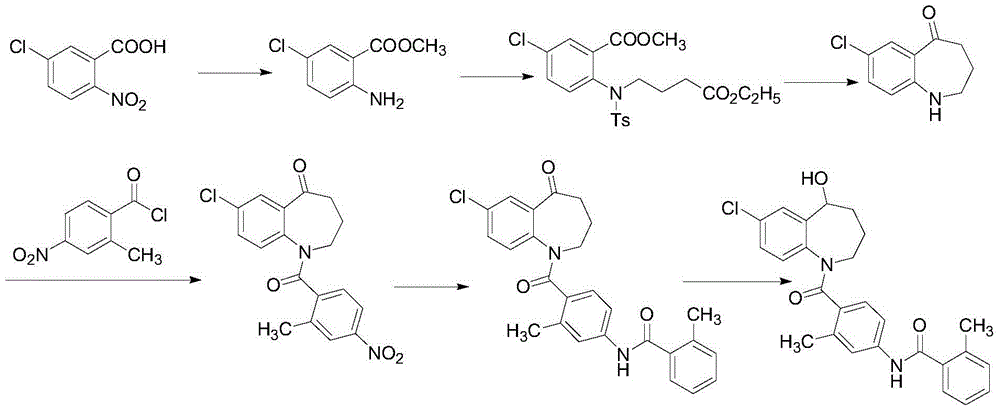
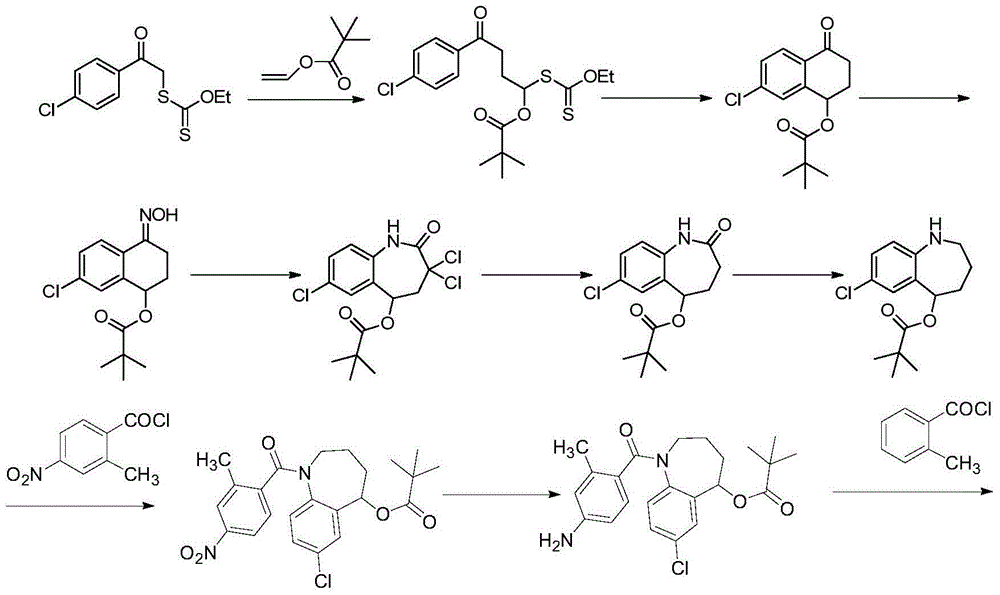
Preparation method of tolvaptan
A technology of tolvaptan and methyl, which is applied in the field of preparation of tolvaptan, a selective non-peptidin arginine vasopressin V2 receptor antagonist, can solve the problem of low yield, difficulty in obtaining starting materials, Reduce the total yield and other problems, achieve high yield and purity, improve work efficiency, and protect the environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] (1) 7-Chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-1-benzazepine Synthesis:
[0070] Add 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine into the reaction flask (5.0g, 25.56mmol) and methanol (50ml), stirred until clear, placed in an ice-water bath to cool to 0 ~ 5 ° C, slowly added sodium borohydride (1.45g, 38.34mmol), stirred for 1h, TLC detection, the reaction complete. Pour the reaction solution into ice water to quench, spin off the methanol under reduced pressure, extract with dichloromethane (30mlx3), combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate by rotary evaporation under reduced pressure to obtain a white solid 7-chloro -5-Hydroxy-2,3,4,5-tetrahydro-1H-1-benzazepine (4.71 g, 93%), MS calculated 197.06, found 197.10.
[0071] (2) Synthesis of 4-amino-2-methylbenzoic acid
[0072] To the suspension of 4-nitro-2-methylbenzoic acid (20.0 g, 110.4 mmol) in concentrated hydrochloric acid (100 ml) and ethano...
Embodiment 2
[0080] (1) 7-Chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-1-benzazepine Synthesis:
[0081] Add 7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine into the reaction flask (5.0g, 25.56mmol) and methanol (50ml), stirred until clear, placed in an ice-water bath to cool to 0 ~ 5 ° C, slowly added sodium borohydride (1.16g, 30.67mmol), stirred for 1h, TLC detection, the reaction complete. Pour the reaction solution into ice water to quench, spin off the methanol under reduced pressure, extract with dichloromethane (30mlx3), combine the organic phases, dry over anhydrous sodium sulfate, filter, and concentrate the filtrate by rotary evaporation under reduced pressure to obtain a white solid 7-chloro -5-Hydroxy-2,3,4,5-tetrahydro-1H-1-benzazepine (4.60 g, 91.1%), MS calcd. 197.06, found 197.10.
[0082] (2) Synthesis of 4-amino-2-methylbenzoic acid
[0083] To the suspension of 4-nitro-2-methylbenzoic acid (10.0 g, 55.2 mmol) in concentrated hydrochloric acid (50 ml) and ethanol (10...
Embodiment 3
[0091] The steps of embodiment 3 and embodiment 1 are basically the same, the only difference is:
[0092] Step (1) 7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-1-benzazepine Lithium aluminum hydride is used to replace sodium borohydride in the synthesis of
[0093] Use palladium / carbon to replace SnCl in the synthesis of step (2) 4-amino-2-methylbenzoic acid 2 2H 2 O;
[0094] Sodium carbonate is used to replace triethylamine in the synthesis of step (3) 2-methyl-4-(2-methylbenzamido)benzoic acid;
[0095] Phosphorus trichloride is used to replace oxalyl chloride in the synthesis of step (4) 2-methyl-4-(2-methylbenzamido)benzoyl chloride;
[0096] In the synthesis of step (5) tolvaptan, triethylamine is used to replace sodium hydroxide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com