Tolvaptan preparation containing micronized tolvaptan and water-soluble auxiliary materials
A water-soluble excipient, tolvaptan technology, applied in the field of tolvaptan preparations, orally disintegrating tablets or orally dissolving tablets, can solve a large number of organic solvents and other problems, and achieve improved dissolution rate, good taste and good absorption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
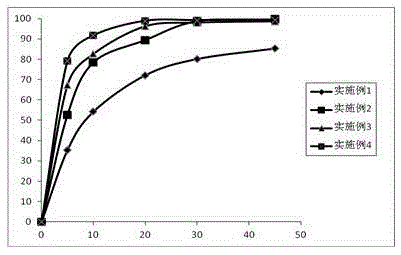
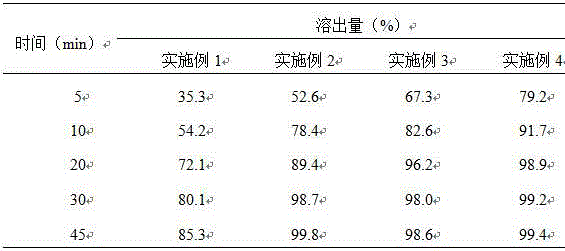
Image
Examples
Embodiment 1
[0025]
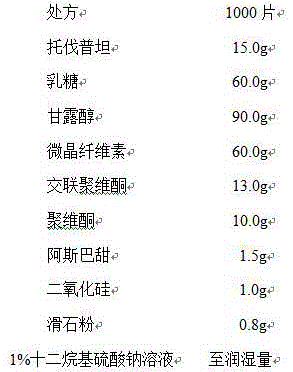
[0026] Process: Mix the prescribed amount of tolvaptan, lactose, microcrystalline cellulose, sucralose, hydroxypropyl cellulose and low-substituted hydroxypropyl cellulose (50%), add 1% lauryl sulfate Sodium solution is used as a wetting agent to make soft materials, granulated with a 20-mesh screen, dried at 55°C, and granulated, adding low-substituted hydroxypropyl cellulose (50%), silicon dioxide and magnesium stearate in the prescribed amount, Mix well and compress into tablets.
Embodiment 2
[0028] Tolvaptan was pulverized until the D90 particle size was not greater than 75 microns, and about 1000 tablets were prepared according to Example 1, each containing 15 mg of tolvaptan.
Embodiment 3
[0030] Tolvaptan was pulverized until the D90 particle size was no more than 50 microns, and about 1000 tablets were prepared according to Example 1, each containing 15 mg of tolvaptan.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com