Clathrates of N-methyl-4-(4-(3-trifluoromethyl)benzoylamino)phenoxyl)nicotinamide and salt thereof, preparation method thereof, and application thereof
A technology of benzamide and pyridine carboxamide, which is applied in the field of medicine, can solve the problems of low solubility and achieve the effects of promoting absorption, improving drug solubility, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
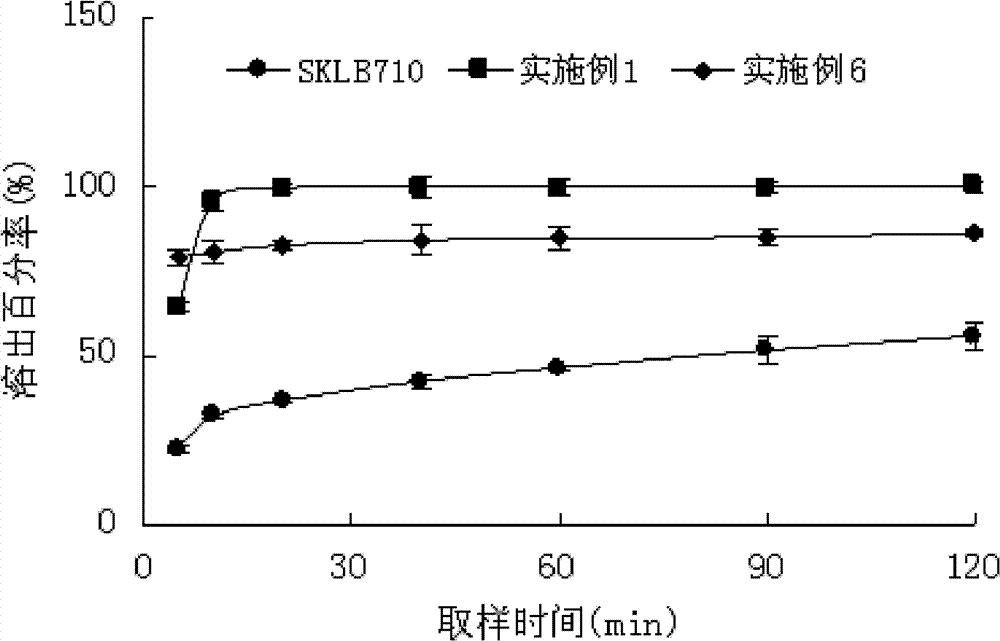
[0039] Take 0.5g of SKLB 710 passed through an 80-mesh sieve, dissolve it in 50ml of ethanol, put it in a mortar with 1g of HP-β-CYD dispersed in water, grind it at a speed of 200rpm, and grind it for 30 minutes; dry it under reduced pressure for 4 hours, pass through a 120-mesh sieve, Instantly. The inclusion rate is 76.5%, and the in vitro dissolution curve is shown in figure 1 . Add lactose, magnesium stearate, sodium carboxymethyl starch, and talcum powder to directly compress into tablets to obtain oral tablets.
Embodiment 2
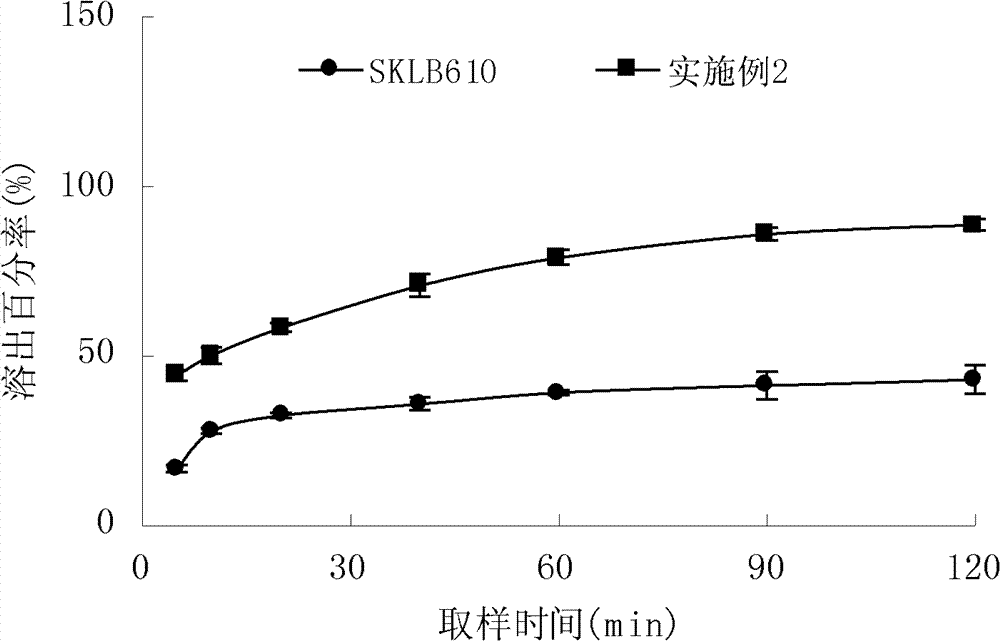
[0041] Take 1 g of SKLB610 passed through an 80-mesh sieve, dissolve it in 50 ml of ethanol, place it in a mortar with 1 g of β-CYD dispersed in water, and grind it at a speed of 200 rpm for 30 minutes. Pass through a 120-mesh sieve. The inclusion rate is 75.4%, and the in vitro dissolution curve is shown in figure 2 . Add lactose or pregelatinized starch, and directly fill capsules.
Embodiment 3
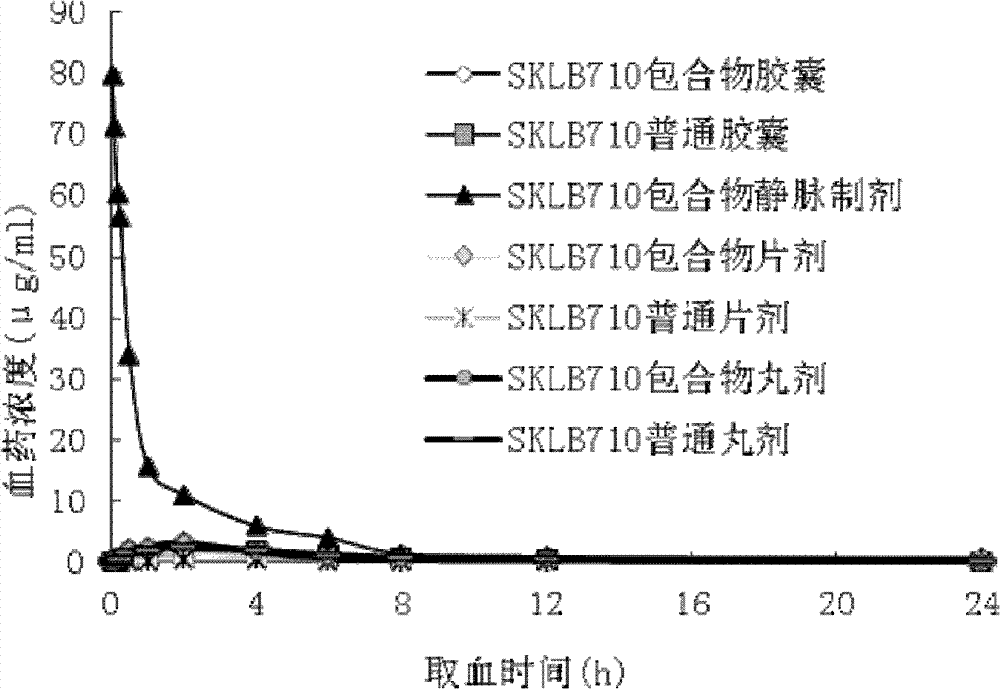
[0043] Add 0.5g of β-CYD and 0.5g of HP-β-CYD into distilled water, stir to dissolve, put them together with 10mg of SKLB 610 in a mortar, and grind at a speed of 100-600rpm for a grinding time of 20-60min. Freeze-drying, the freeze-drying time is 24h. Grind again and pass through a 200-mesh sieve to obtain the clathrate. The inclusion rate is 89%. The clathrate can be directly dissolved in water, which can increase the solubility of SKLB610 raw material drug in water (0.00098mg / ml) by nearly 1000 times. Add mannitol to adjust isotonicity, and then use a 0.22μm microporous membrane to sterilize, pack, Freeze-dried to obtain freeze-dried powder for injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com