Tolvaptan intermediate and preparation method thereof
A technology for tolvaptan and an intermediate, which is applied in the field of medicinal chemistry, can solve environmental problems, expensive metal salt by-products and other problems, and achieves the effects of cheap reagents, easy operation and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
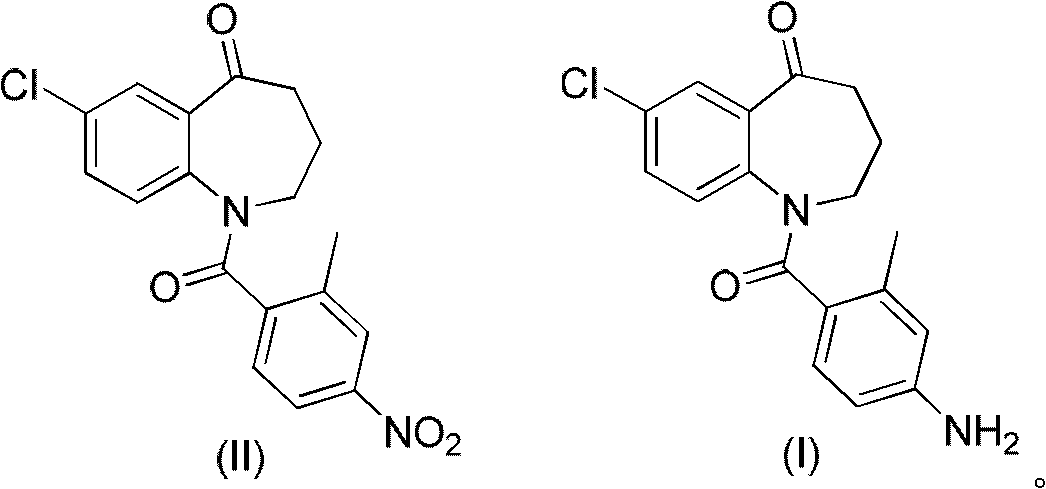
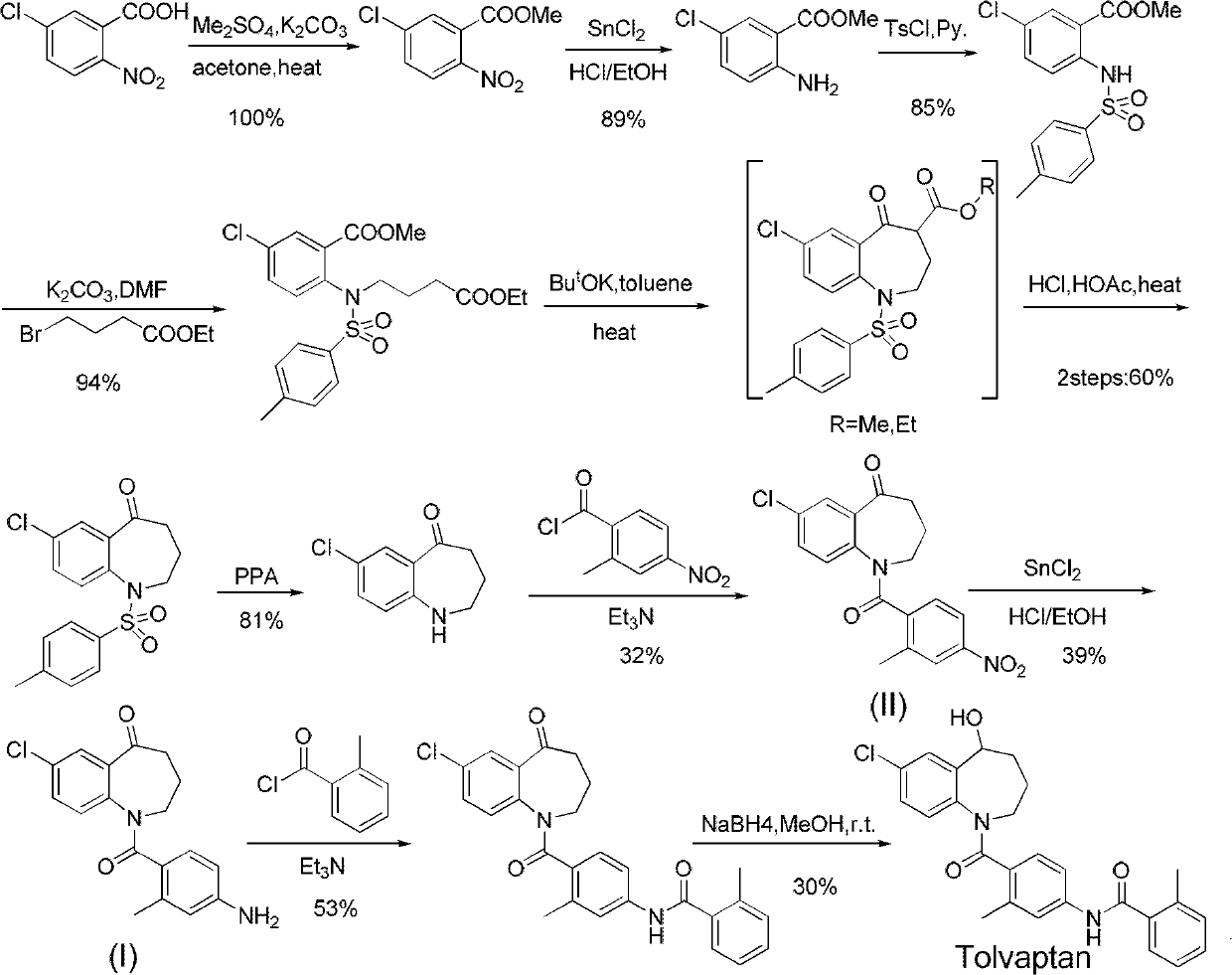
[0033] Embodiment 1: 1-(4-amino-2-methylbenzoyl)-7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine ( Preparation of formula (I) compound)
[0034] In a 100mL four-neck round bottom flask equipped with a magnetic stirrer and a thermometer, add 7-chloro-1-(2-methyl-4-nitrobenzoyl)-5-oxo-2,3,4, 5-tetrahydro-1H-1-benzazepine (compound of formula (II)) 4.70g, iron powder 1.60g, 98% concentrated sulfuric acid 4.20g, ammonium sulfate 20.0g, ethanol 50mL. React at 60°C for 5 hours, TLC shows that the reaction is complete, then filter, wash the filter cake with ethanol, combine the filtrates and then rotary evaporate under reduced pressure, add 50 mL of dichloromethane to the obtained product, slowly add saturated sodium bicarbonate solution dropwise, adjust the pH to 7-8, and divide After the liquid, the organic phase was washed with brine, dried over anhydrous magnesium sulfate, filtered and evaporated to remove the solvent to obtain 4.10 g of the compound of formula (II) as a pale...
Embodiment 2
[0037] Embodiment 2: 1-(4-amino-2-methylbenzoyl)-7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine ( Preparation of formula (I) compound)
[0038] In a 100mL four-neck round bottom flask equipped with a magnetic stirrer and a thermometer, add 7-chloro-1-(2-methyl-4-nitrobenzoyl)-5-oxo-2,3,4, 4.70 g of 5-tetrahydro-1H-1-benzazepine (compound of formula (II)), 1.90 g of zinc powder, 4.50 g of 98% concentrated sulfuric acid, 20.0 g of ammonium sulfate, and 50 mL of ethanol. React at 60°C for 4 hours, filter after TLC shows that the reaction is complete, wash the filter cake with ethanol, combine the filtrates, and then rotary evaporate under reduced pressure. After the liquid, the organic phase was washed with brine, dried over anhydrous magnesium sulfate, filtered and evaporated to remove the solvent to obtain 4.00 g of the compound of formula (II) as a pale yellow foamy solid, mp 190-191°C, yield 93.8%.
Embodiment 3
[0039] Embodiment 3: 1-(4-amino-2-methylbenzoyl)-7-chloro-5-oxo-2,3,4,5-tetrahydro-1H-1-benzazepine ( Preparation of formula (I) compound)
[0040] In a 100mL four-neck round bottom flask equipped with a magnetic stirrer and a thermometer, add 7-chloro-1-(2-methyl-4-nitrobenzoyl)-5-oxo-2,3,4, 2.0 g of 5-tetrahydro-1H-1-benzazepine (compound of formula (II)), 0.7 g of iron powder, 2.5 g of 70% concentrated sulfuric acid, 8.5 g of ammonium sulfate, and 25 mL of ethanol. React at 60°C for 4 hours, filter after TLC shows that the reaction is complete, wash the filter cake with ethanol, combine the filtrates, and then rotary evaporate under reduced pressure. The organic phase was washed with brine, dried over anhydrous magnesium sulfate, filtered and evaporated to remove the solvent to obtain 1.44 g of the compound of formula (II) as a light yellow foamy solid, mp 190-191°C, yield 79.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com