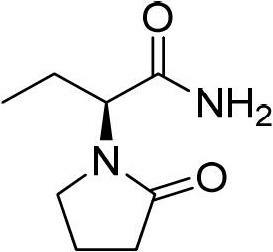
Preparation method of levetiracetam
A technology of aminobutanamide and amide, which is applied in the field of compound preparation, can solve the problems of complicated reaction operation, high cost, unsatisfactory product purity, etc., and achieves the effects of reducing environmental pollution and cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
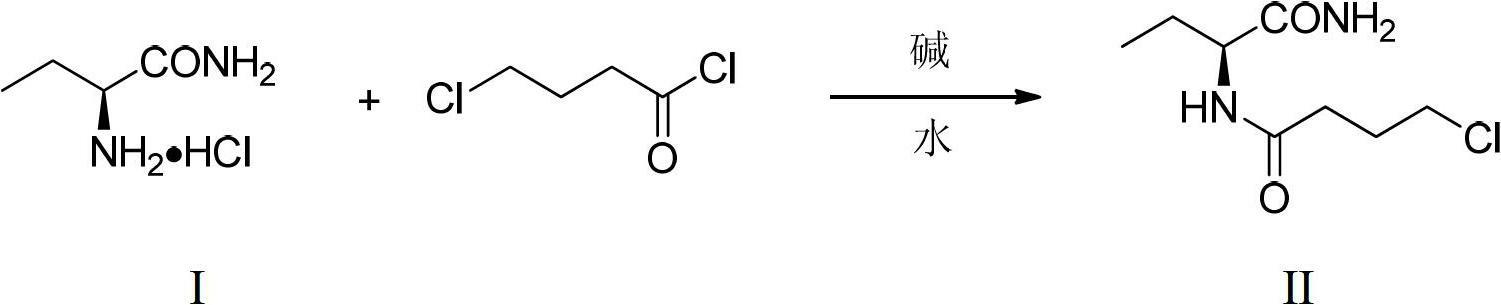
[0030] (1) Synthesis of (S)-N-(1-amino-1-oxo-2-butyl)-4-chlorobutanamide
[0031] Mix α-aminobutyramide hydrochloride (3g, 21.65mmol) and water (15ml), add triethylamine (7.55ml, 54.13mmol), add 4-chlorobutyryl chloride (3.15ml, 28.14mmol) dropwise under stirring ), after the dropwise addition, continue to stir for 1h. Add an appropriate amount of sodium chloride and stir, and a solid precipitates out. Suction filter, and wash the solid with a small amount of 5ml ethyl acetate. Gained solid weighs 3.57g in air, productive rate 79.8%, melting point 118-123 DEG C, α D 25 = -22.3° (c=1, methanol). 1 HNMR (CDCl 3 , 400MHz): δ0.96(t, J=7.5Hz, 3H), 1.64-1.71(m, 1H), 1.87-1.92(m, 1H), 2.10-2.15(m, 2H), 2.40-2.45(m ,2H),3.60(t,J=6.4Hz,2H),4.43(q,J=6.8Hz,1H),5.62(br,1H),6.24(br,1H),6.34(d,J=7.4Hz ,1H).
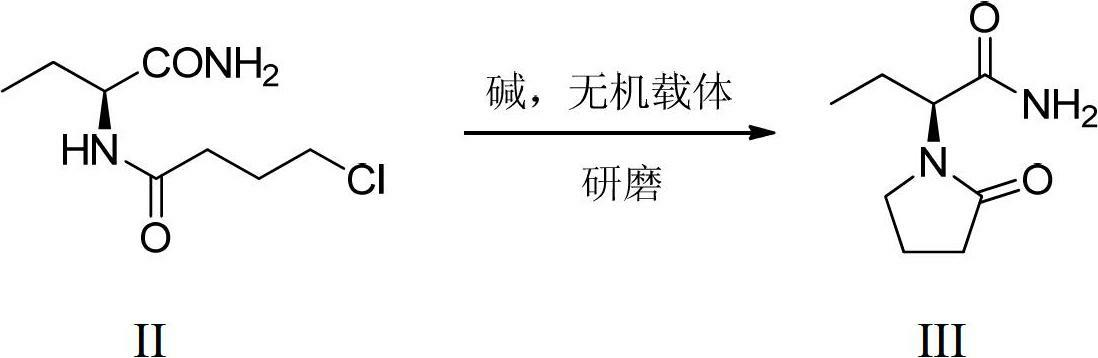
[0032] (2) Synthesis of Levetiracetam
[0033] Potassium hydroxide (0.17 g, 2.18 mmol) and 1 g of anhydrous sodium sulfate were mixed and ground in a mortar for 3 min. Add (S)...
Embodiment 2
[0035] (1) Synthesis of (S)-N-(1-amino-1-oxo-2-butyl)-4-chlorobutanamide
[0036] Mix α-aminobutyramide hydrochloride (3g, 21.65mmol) and water (9ml), add sodium bicarbonate (5.64g, 67mmol), add dropwise 4-chlorobutyryl chloride (4.85ml, 43.3mmol) under stirring , Continue to stir for 1h after the dropwise addition. Suction filtration, the solid was washed with a small amount of ethyl acetate. After drying in air, 3.45 g of solid was obtained, with a yield of 77.2%.
[0037] (2) Synthesis of Levetiracetam
[0038] Potassium hydroxide (0.29 g, 7.26 mmol) and 3 g of anhydrous sodium sulfate were mixed and ground in a mortar and pestle for 3 min. (S)-N-(1-amino-1-oxo-2-butyl)-4-chlorobutanamide (1.00 g, 4.84 mmol) was added, and the grinding was continued for 15 min, followed by TLC until the reaction was complete. Heat to dissolve with ethyl acetate, cool after hot filtration, crystals are precipitated, and 0.63g product is obtained by suction filtration, yield 76.8%, meltin...
Embodiment 3
[0040] (1) Synthesis of (S)-N-(1-amino-1-oxo-2-butyl)-4-chlorobutanamide
[0041] Mix α-aminobutyramide hydrochloride (3g, 21.65mmol) and water (9ml), add potassium hydroxide (3.64g, 64.95mmol), add 4-chlorobutyryl chloride (4.85ml, 43.3mmol) dropwise under stirring ), and continued to stir for 1 h after the dropwise addition. Suction filtration, the solid was washed with a small amount of ethyl acetate. After drying in air, 3.51 g of solid was obtained, with a yield of 78.5%.
[0042] (2) Synthesis of Levetiracetam
[0043] Same as the second step of Example 2, obtain 0.60g product, productive rate 73.2%, 115.6-117.2 ℃ of fusing points, α D 25 =-81.2° (c=2, water).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com