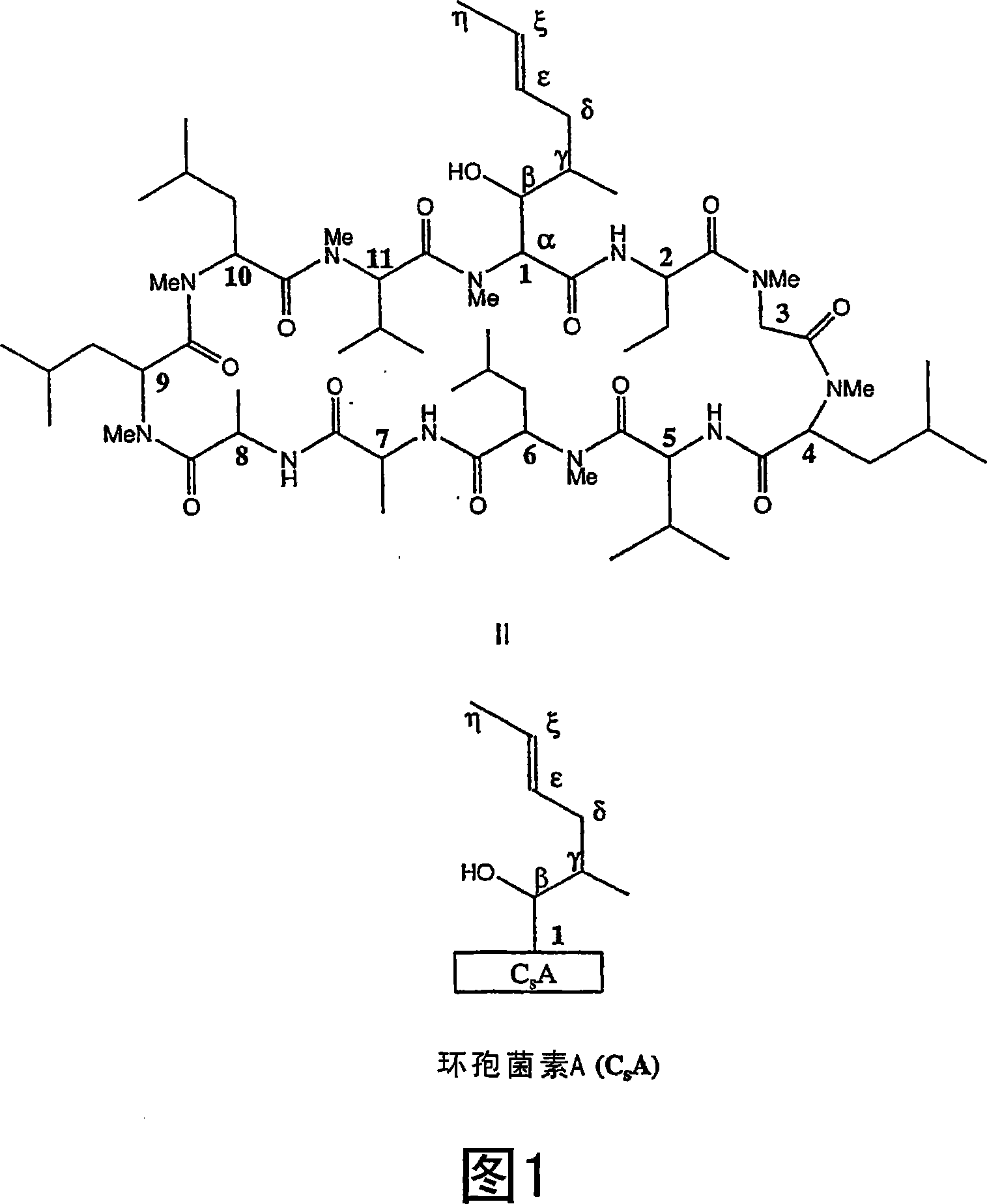
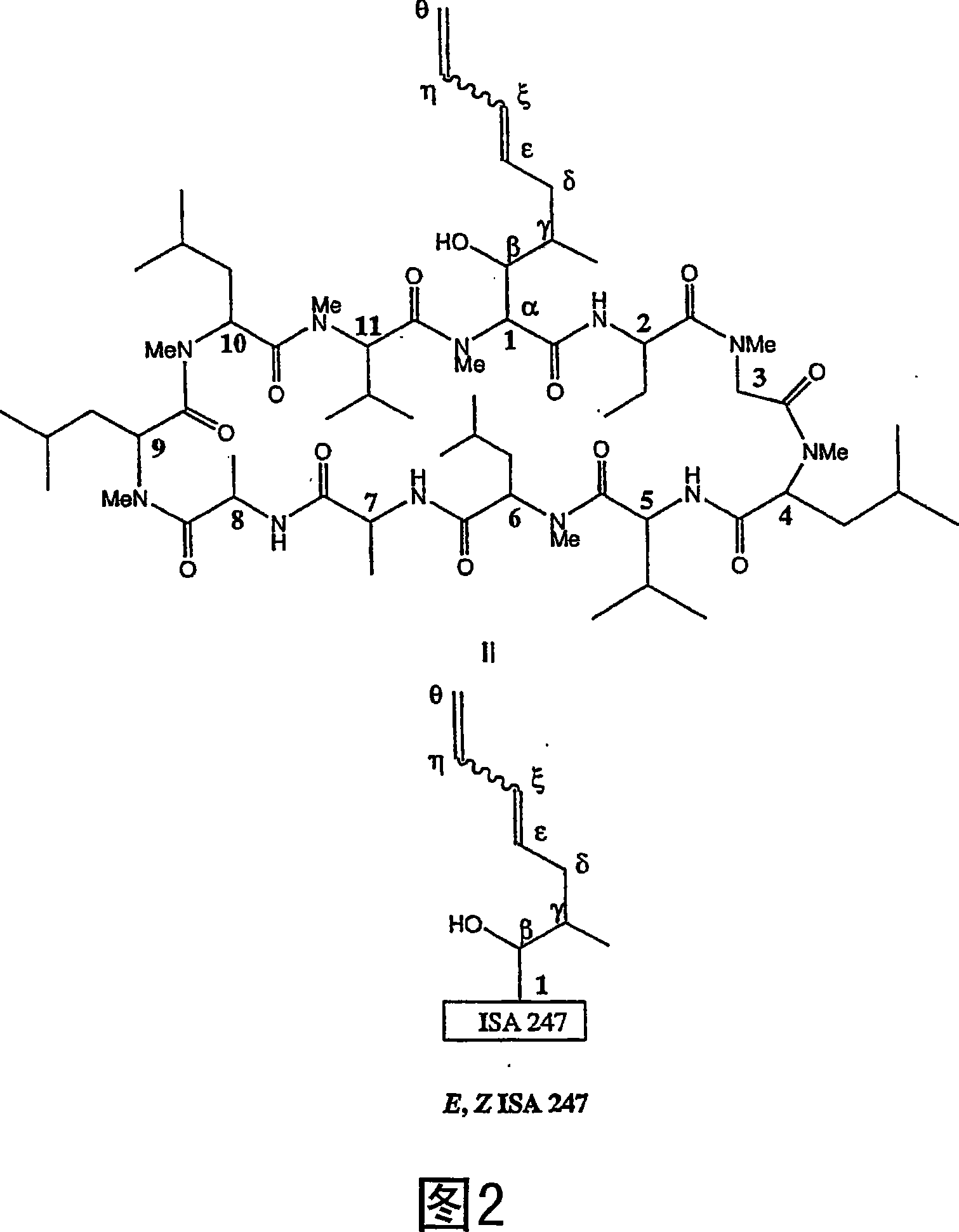
Metabolites of cyclosporin analogs
A metabolite, representative technology for the manufacture and analysis of isolated ISA247 metabolites to address issues such as reducing toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0335] Metabolites of ISA247 can be produced using chemical synthesis methods. The chemical synthesis of these metabolites generally follows the following steps: 1) protection of the β-OH of the 1 amino acid side chain of cyclosporin A or ISA247; 2) epoxidation; 3) diol formation and 4) deprotection. Although the protection of β-OH is favorable for the formation of epoxide and diol metabolites, it is not suitable for the formation of cyclic metabolites. Possible protecting groups at the β-OH include acetyl, trimethylsilyl, benzoate, substituted benzoate, ether and methylsilyl ether. Under certain reaction conditions, the acetate protecting group is prone to cause undesired side reactions such as elimination and hydrolysis. Since benzoates, ethers and silyl ethers are generally more resistant to such side reactions under the same reaction conditions, it is often advantageous to use these protecting groups instead of acetates.
[0336]Under basic conditions, protected CsA or I...
Embodiment 1
[0343] Example 1. Preparation of ISA247 metabolites from whole blood
[0344] Whole blood was drawn from humans after ISA247 administration. ISA247 and its metabolites were extracted from whole blood using tert-butyl-methyl-ether (or methyl tert-butyl ether, MTBE), dried and redissolved in methanol. 2 mL of MTBE (cat. No. 7001-2; Caledon) was added to 200 uL of blood, shaken for 10 minutes, and centrifuged for 2 minutes in a desktop centrifuge. The upper MTBE layer was removed and concentrated in vacuo. The residue was redissolved in 200uL methanol. Bile and urine extractions were performed similarly.
Embodiment 2
[0345] Example 2: Chemical Synthesis of ISA247 Metabolites
[0346] Preparation of monoepoxides of OAc-E-ISA247
[0347] To prepare the diol metabolites of E-ISA247, epoxides were formed as shown in FIG. 42 . Follow the steps below. To CHCI of OAc-E-ISA247 (125 mg, 0.1 mmol) which was homogenized and cooled (0 °C) 3 (3 mL) was added potassium bicarbonate (10 mg) to the solution. Then m-chloroperbenzoic acid (23 mg, 0.1 mmol, 77%) in CHCI was added 3 (2 mL) solution. The reaction mixture was warmed to room temperature and stirring was continued for 18 hours. The reaction product was extracted with dichloroethane (25 mL). with saturated NaHCO 3 solution and brine to wash the organic layer. dry (Na 2 SO 4 ) and solvent removal gave a white solid (110 mg). MS (m / z): 1295 (M+Na+). The product is a mixture of epoxides. The same method can be used with OAc-Z-ISA247 or a mixture of ISA247 isomers, but the stereochemistry of the products will be different, as shown in Fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com