Polymer containing azobenzene circlet on side chain and preparation method thereof
A technology for azobenzene and polymers, applied in the field of preparation of polymers containing azobenzene small rings in the side chain, to achieve the effects of controllable molecular weight, clear structure and narrow molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
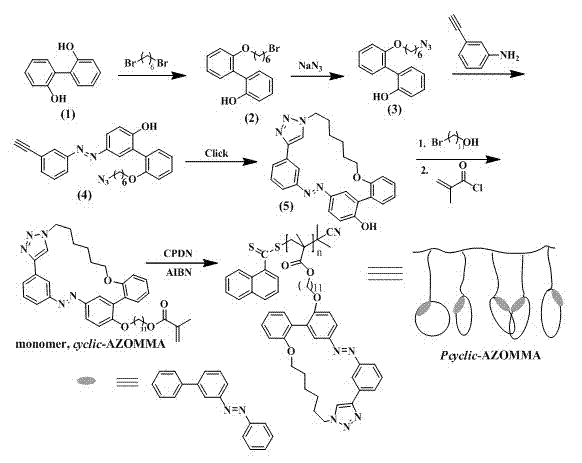
[0030] refer to figure 1Shown, a kind of side chain contains the polymkeric substance of azobenzene small ring and preparation method, comprises the following steps:
[0031] Step 1) Take compound The compound is obtained by etherification with 1,6-dibromohexane and azidation with sodium azide , (3) through the coupling reaction with the diazonium salt of m-aminophenylacetylene to obtain the compound , (4) Cyclic azo compounds were obtained by the intramolecular Click reaction of the azide / alkynyl CuAAC method , (5) Etherification with bromoundecyl alcohol and esterification with methacryloyl chloride to obtain methyl methacrylate (MMA) monomer containing azobenzene small ring cyclic- AZOMMA, its structural formula is:
[0032] ;
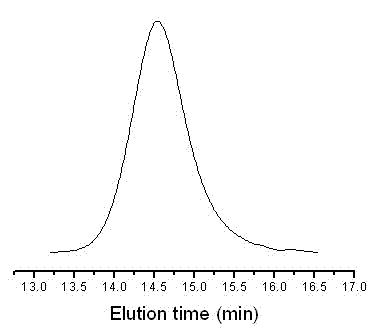
[0033] Step 2) Living radical polymerization of monomers by reversible addition-fragmentation transfer (RAFT); the polymerization system includes monomers, RAFT reagents, and free radical initiators; wherein, in molar ratio, [monomer] ...
Embodiment 1
[0039] 1. If figure 1 As shown, the synthesis of the monomer mainly has the following steps:
[0040] Step 1) Add 2.0g 2,2 to a 500mL three-neck flask , -Dihydroxybiphenyl, 2.97g K 2 CO 3 , 300mL of acetone, 70 o C oil bath was heated to reflux. Add 2.8 g of 1,6-dibromohexane in acetone solution dropwise into the reaction system. React for half an hour after dropping. The reaction solution was suction filtered, the filtrate was rotary evaporated to remove the solvent, and the crude product was purified by column chromatography to obtain compound (2).
[0041] Step 2) Add 4.0g compound (2), 1.13g NaN to a 50mL round bottom flask 3 , and after 30mL DMF at 50 o C oil bath was reacted for 24 hours. After the reaction was completed, the reaction solution was poured into ethyl acetate, washed with water three times, and the organic phase was collected, and the solvent was removed by rotary evaporation to obtain compound (3).
[0042] Step 3) Add 4.25g m-aminophenylace...
Embodiment 2
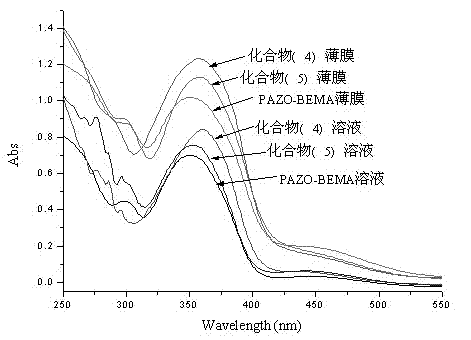
[0049] Embodiment two: compound (4), (5) and homopolymer P cycle -UV-Vis absorption spectrum of AZOMMA
[0050] Weigh compound (4) and compound (5) 0.554mg, homopolymer P cycle -AZOMMA 0.225mg was prepared into 10mL tetrahydrofuran solution for use, and the concentration of azophenyl groups in the solution was 5×10 -5 mol / L. The above solutions were respectively dropped on the quartz glass plate to dry naturally to form a thin film for use. The above solution and film were tested for UV-Vis absorption, such as image 3 . It was found that relative to the solution state, the maximum absorption peak of compound (5) had a red shift of 5nm in the thin film, but this phenomenon was not observed for compound (4) and homopolymer.
[0051] The above solution of compound (4) was irradiated with 365nm ultraviolet light for different time to measure the change of its ultraviolet-visible absorption, such as Figure 4 . Due to the existence of the azobenzene structure, the trans-c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mn | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com