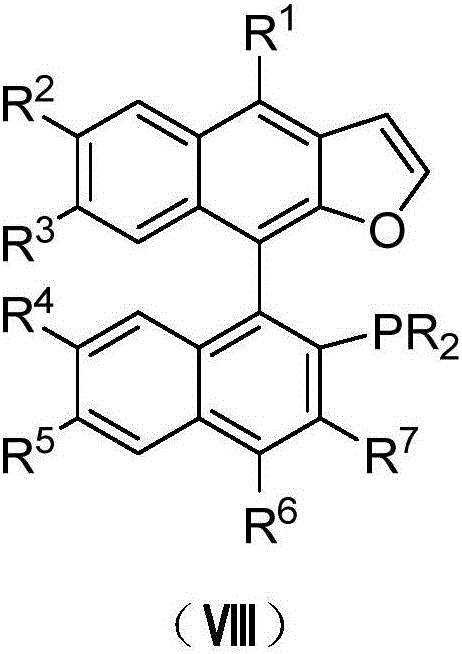
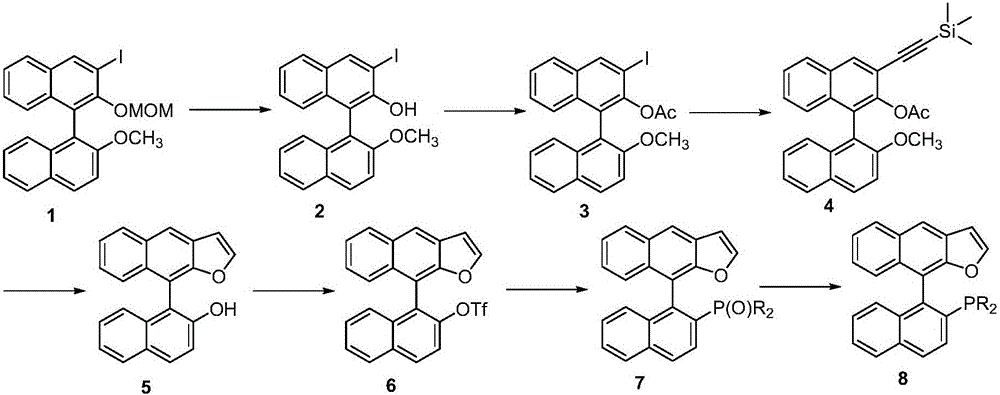
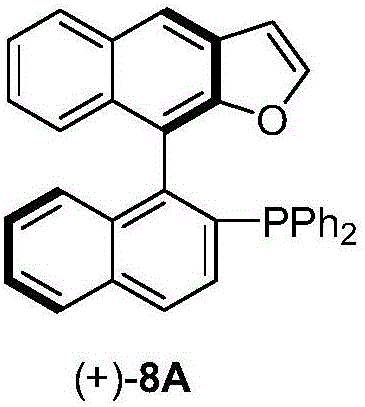
Naphthofuran-structure-containing biaryl monophosphine ligands, and preparation method and application thereof
A kind of technology of biaryl mono-phosphine ligand, applied in the field of biaryl monophosphine ligand and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1 biaryl monophosphine ligand
[0049] In this embodiment, the biaryl monophosphine ligand represented by the formula ((+)-8A) is taken as an example to introduce its preparation process.
[0050]
[0051] (1), use compound shown in formula ((+)-1) to prepare compound shown in formula ((+)-2)
[0052]
[0053]Under the protection of nitrogen, dissolve 1.0g (2.13mmol) of the compound represented by formula ((+)-1) in 50mL of ethanol, add 10mL of 4mol / L dilute hydrochloric acid, react at 80°C for 7h, spin dry the ethanol under reduced pressure, and then add Dissolve in 100mL of water, extract 3 times with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous magnesium sulfate, and remove the solvent under reduced pressure to obtain 850mg of product formula ((+)-2) The shown compound, yield: 93%, product analysis result: [α] D 17 +47.5 (c=0.1, CHCl 3 ), 1 H NMR (400MHz, CDCl 3 )δ8.48(1H,s),8.10(1H,d,J=12.0Hz),7.93(1...
Embodiment 2
[0076]
[0077] In the glove box, Sm1 (0.1mmol, 1.0equiv), Sm2 (0.2mmol, 2.0equiv), K 3 PO 4 (0.3mmol, 3.0equiv), Pd 2 (dba) 3 (1.0% mmol), (±)-8A (2.0% mmol) and toluene (4.0 mL) were placed in a 25 mL one-necked bottle, and stirred at 80° C. for 1 hour under nitrogen protection. Product result analysis: 1 H NMR (400MHz, CDCl 3 )δ8.05-8.01(3H,m),7.95(1H,d,J=8.0Hz),7.69(1H,t,J=4.0Hz),7.52-7.49(3H,m),7.40-7.23(5H ,m),3.81(3H,s). 13 C NMR (101MHz, CDCl 3 )δ154.6, 134.6, 134.3, 133.7, 133.0, 129.5, 129.0, 128.5, 128.2, 127.8, 126.2, 125.7, 125.6, 125.5, 123.6, 123.2, 113.8, 56.8.
Embodiment 3
[0079]
[0080] In the glove box, Sm1 (0.1mmol, 1.0equiv), Sm3 (0.2mmol, 2.0equiv), K 3 PO 4 (0.3mmol, 3.0equiv), Pd 2 (dba) 3 (1.0% mmol), (±)-8A (2.0% mmol) and isopropanol (4.0 mL) were placed in a 25 mL one-necked bottle, and stirred at 80° C. for 4 hours under nitrogen protection. Product result analysis: 1 H NMR (400MHz, CDCl 3 )δ8.70-8.68 (1H, m), 8.66 (1H, s), 7.97 (1H, d, J = 12.0Hz), 7.87 (1H, t, J = 4.0Hz), 7.76-7.73 (1H, m ),7.50-7.45(2H,m),7.42-7.38(3H,m),3.87(3H,s). 13 C NMR (101MHz, CDCl 3 )δ154.1, 151.8, 148.2, 138.6, 133.3, 130.0, 128.9, 128.1, 126.8, 124.5, 123.7, 123.1, 133.3, 56.5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com