Fluorescent probe for detecting biological mercaptan in lysosome and preparation method and application of fluorescent probe
A technology of fluorescent probes and biothiols, which is applied in the field of analytical chemistry to achieve the effects of low preparation cost, fast response and easy promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
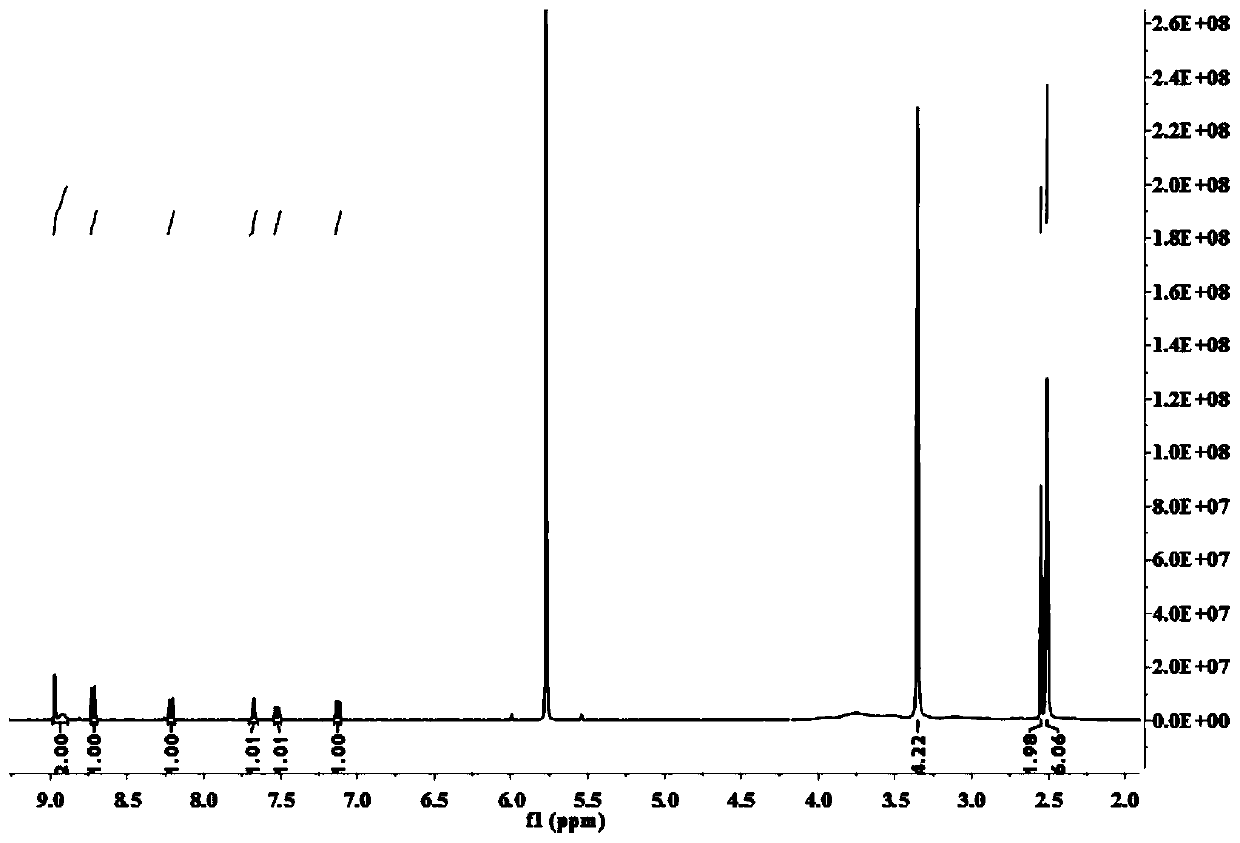
[0033] Example 1 Synthesis of Fluorescent Probe CM-NBD
[0034] (1) Compound a1 (1 mmol), a2 (1 mmol), 1-hydroxybenzotriazole (0.5 mmol), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide ( 3 mmol) into a 25 mL single-necked flask, dissolved with N,N-dimethylformamide (5 mL), stirred at room temperature for 10 min, and the catalyst N,N-diisopropylethylamine (0.1 mL) was slowly Add dropwise into the flask and stir at room temperature for 5h. After the reaction, use water-dichloromethane to extract, and the compound (I) obtained after the lower organic phase is spin-dried:
[0035] ;
[0036] (2) Compound (I) (0.36 mmol) was stirred with a3 (0.36 mmol) and potassium carbonate (0.3 mmol) in N,N-dimethylformamide (3 mL) at room temperature for 5 h. After the reaction was complete, filter , the filtrate was spin-dried by distillation under reduced pressure, and purified by column chromatography with dichloromethane:methanol (v / v)=25:1 as the chromatographic solution to obtain a ye...
Embodiment 2
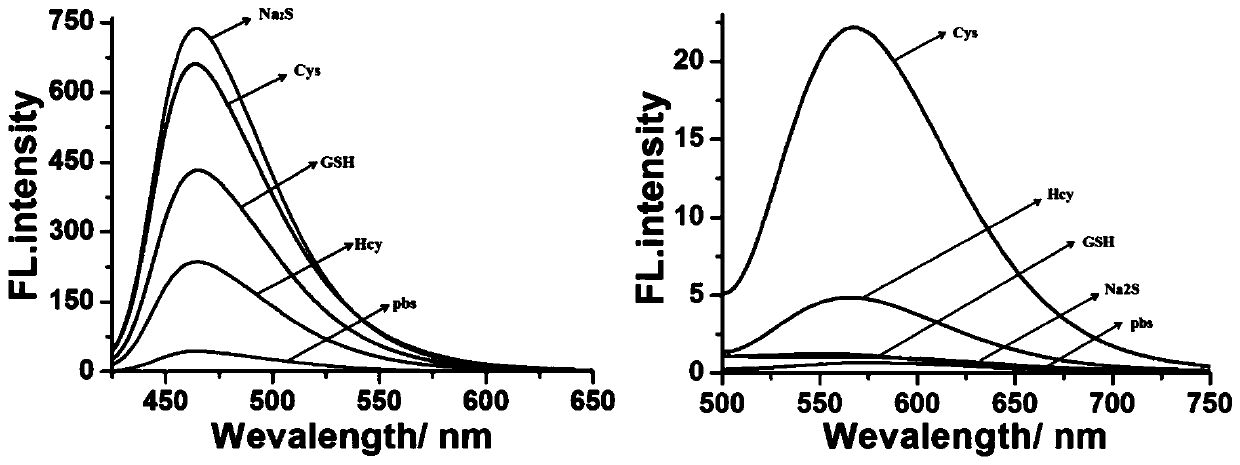
[0039] Example 2 Fluorescence spectra of fluorescent probe CM-NBD under different biothiols
[0040] Prepare 1 mM DMSO mother solution of the fluorescent probe CM-NBD obtained in Example 1 for future use. Prepare 5 parts of 2 mL PBS buffer solution (containing 20% DMSO, pH=5) in advance, add 20 μL of probe mother solution, and add 20 μL of 10 mM biothiol (Cys, Hcy, GSH, Na 2 S). Fluorescence detection is then performed (λ ex =405 nm and 470 nm), calculate the fluorescence intensity in each system, the results are as follows figure 2 shown. Depend on figure 2 It can be seen that in the presence of Cys and Hcy, the probe produces two different fluorescence emissions at 465 nm and 565 nm under the excitation of two independent wavelengths. However, adding Na to the probe 2 S and GSH will only fluoresce blue at 465 nm. It indicated that the fluorescent probe CM-NBD could be used to detect biothiols.
Embodiment 3
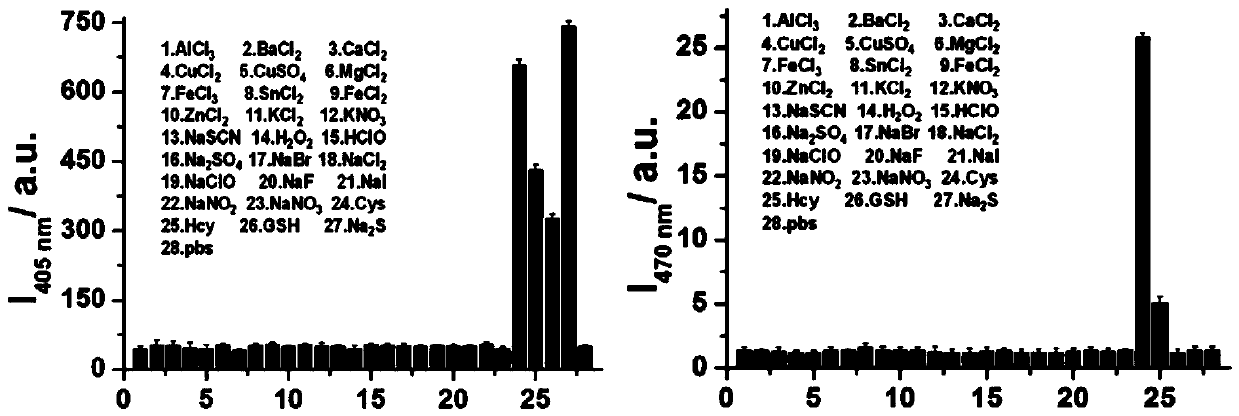
[0041] Example 3 Selectivity of Fluorescent Probe CM-NBD
[0042] Prepare 1 mM DMSO mother solution of the fluorescent probe CM-NBD obtained in Example 1 for future use. Prepare 28 copies of 2 mL fluorescent probe CM-NBD buffer solution (containing 20% DMSO, pH=5) obtained in Example 1 in advance, add 20 μL of the probe mother solution to each, and then add 20 μL of the concentration of 10 mM AlCl 3 , BaCl 2 , CaCl 2 , CuCl 2 、CuSO 4 , MgCl 2 , FeCl 3, SnCl 2 , FeCl 2 , ZnCl 2 , KCl 2 、KNO 3 , NaSCN, H 2 o 2 , HClO, Na 2 SO 4 , NaBr, NaCl 2 , NaClO, NaF, NaI, NaNO 2 、NaNO 3 , Cys, Hcy, GSH, Na 2 S in PBS solution. Fluorescence detection is then performed (λ ex =405 nm and 470 nm); calculate the fluorescence intensity in each system, as shown in 3. Depend on image 3 It can be seen that the selectivity of the fluorescent probe to biothiol is much higher than that of other substances, indicating that the fluorescent probe has the characteristic of sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com