Novel asparaginases and uses thereof
a technology of asparaginase and polypeptide, which is applied in the field of newly identified asparaginase polypeptide variants and to polynucleotide sequences comprising genes, to achieve the effect of significantly increasing the expression of a variant asparaginase according to the invention and increasing the activity of the produced asparaginas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Fermentation, Isolation and Purification of Asparaginases According to the Invention
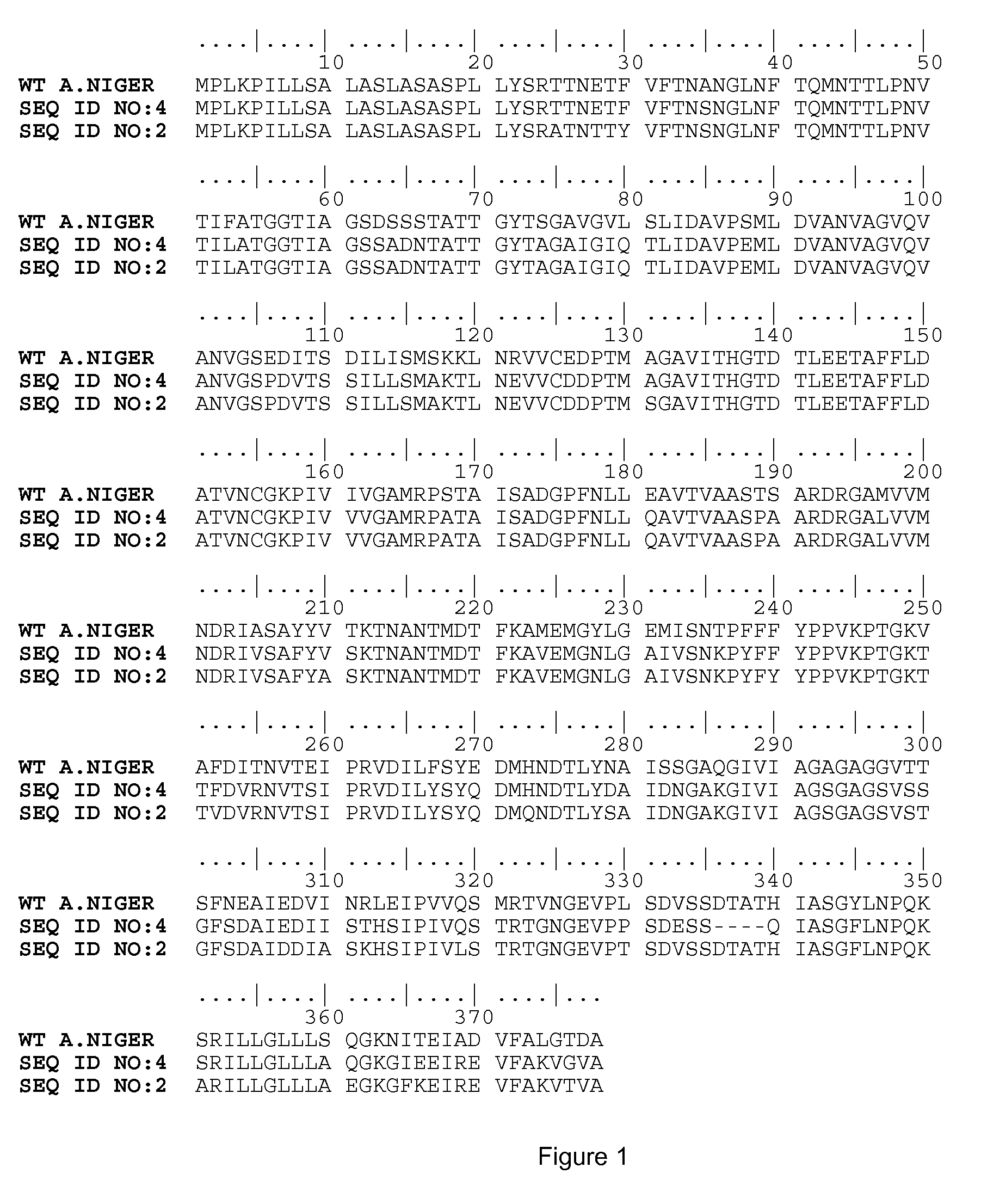
[0347]Asparaginases of the invention were obtained by the construction of expression plasmids containing a DNA sequence encoding the asparaginase of the invention, transforming an Aspergillus niger strain with the plasmid and growing the Aspergillus niger strains as described in WO2004 / 030468.
[0348]After growing Aspergillus niger containing the proper expression plasmids cell free supernatants were prepared by centrifugation of the fermentation broth at 5000×g for 30 minutes at 4° C. If necessary the supernatants were filtered further over a Miracloth filter (Calbiochem cat #475855) and a GF / A Whatmann Glass microfiber filter (150 mm {acute over (Ø)}), respectively, to remove any solids. To remove any fungal material the supernatants could be adjusted to pH=5 with 4N KOH and sterile filtrated over a 2 μm (bottle-top) filter with suction. The supernatants were stored until use at 4° C. or frozen at −2...
example 2
Performance of Asparaginase According to the Invention
[0351]Specific Activity as a Function of pH
[0352]The specific activity of the asparaginase variants was determined at pH=4, pH=5, pH=6, pH=7, pH=8 at 37° C. in 50 mM phosphate / citrate buffer using cell-free supernatants. Specific enzyme activity, is herewith defined as the activity determined for an enzyme sample in units / mg of pure enzyme polypeptide. Specific asparaginase activity is therefore the activity determined for an asparaginase sample in units / mg of pure asparaginase polypeptide.
TABLE 1The specific activity of the variants relative to wild type (wt) A. nigerasparaginase (WO2004 / 030468) using asparagine as a substrateat the indicated pH values. For each pH the wild type specific activityis set to 100%. Activity was determined at 37° C.pH = 4pH = 5pH = 6pH = 7pH = 8(%)(%)(%)(%)(%)wt100100100100100ASN002141252263741033ASN001552464186971758
[0353]For determination of the specific activity the asparaginase, concentration in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com