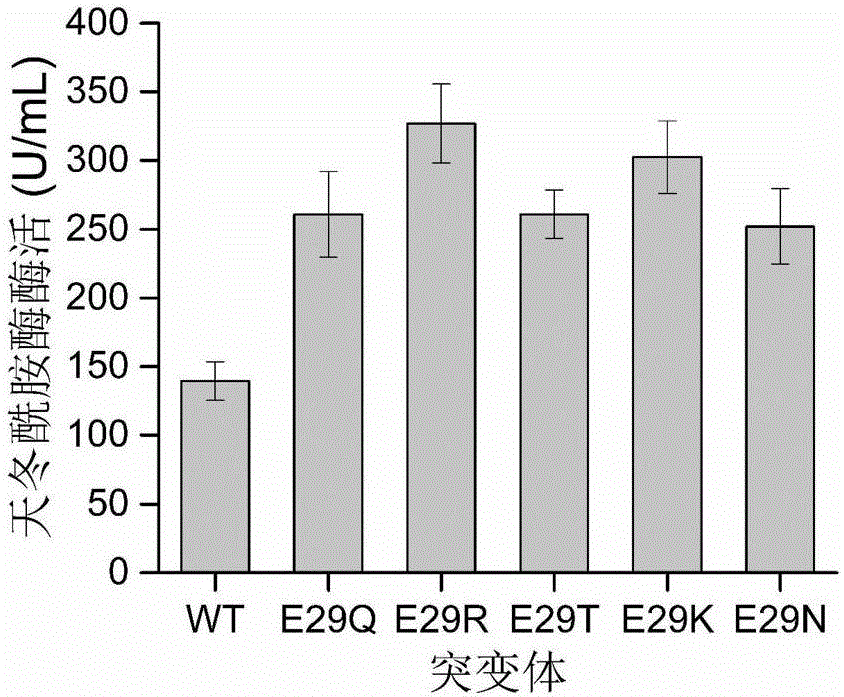
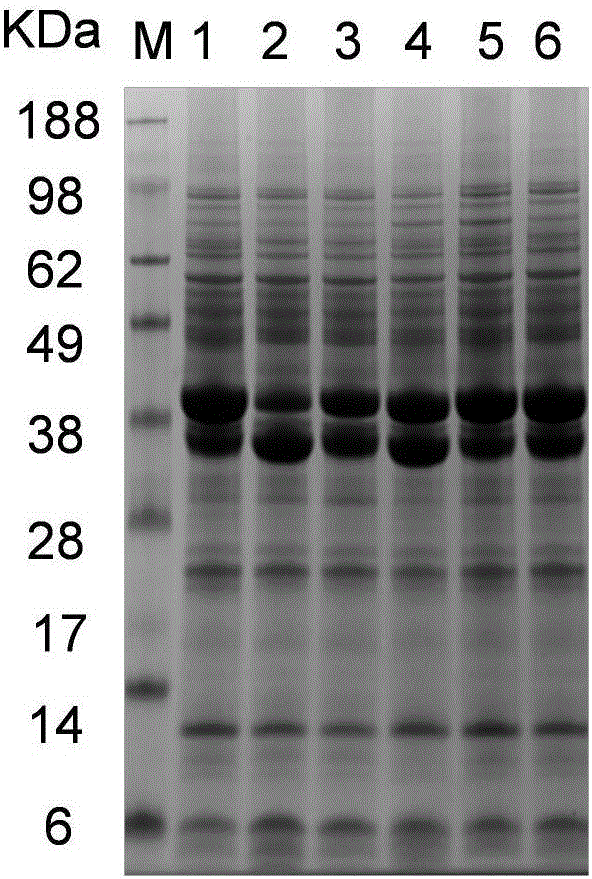
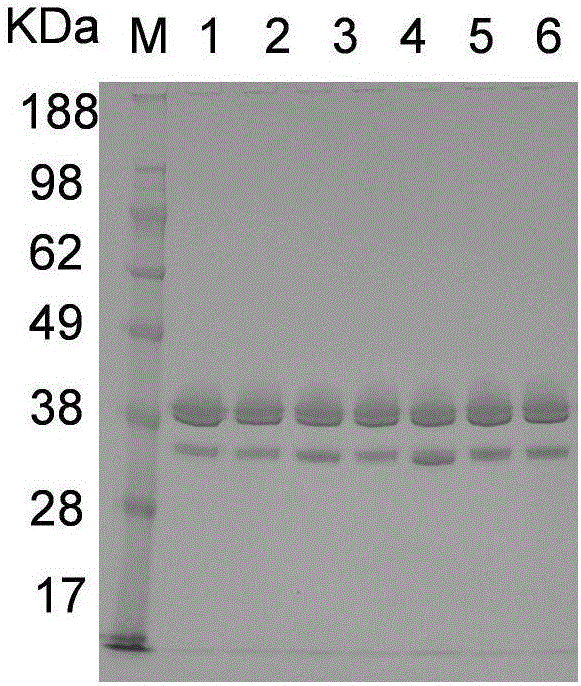
Asparaginase mutant with improved enzyme activity
An asparaginase and asparagine technology, applied in the field of enzyme engineering, can solve the problem of low yield and the like, and achieve the effects of increasing yield, reducing production cost and improving catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Simulation of crystal structure of asparaginase derived from Bacillus subtilis
[0031] Using the reported Erwinia chrysanthemi asparaginase (PDB code: 1hg0) as a template (Structural basis for the activity and substrate specificity of Erwinia chrysanthemi L-Asparaginase, published in 2001) (the amino acid similarity between the two is 58.1%), using online simulation The software SWISS-MODEL simulates the crystal structure of asparaginase derived from Bacillus subtilis.
Embodiment 2
[0032] Embodiment 2 site-directed mutation E29 asparaginase strain construction
[0033] The designed upstream and downstream primers P1 and P2 (as shown in Table 1) were used to carry out PCR using the pP43H-D30 plasmid as a template to construct an E29 saturation mutant strain. The PCR conditions are: 98°C for 3min, 98°C for 30S, 55°C for 90S, 72°C for 8min, 34 cycles. PCR amplification system: template 1 μL, upstream and downstream primers 1 μL, dNTP Mix 4 μL, 5×primeSTARBuffer 10 μL, sterilized double distilled water 32.5 μL, prime STAR DNA polymerase 0.5 μL. The gel recovery kit was used to purify and recover the PCR product, and the concentration of the recovered product was checked by electrophoresis. DpnI digested and processed PCR recovered product, transformed it into competent E.coil JM109, and picked positive colony with ampicillin LB plate. After culturing on a shaker overnight at 37°C, the plasmid was extracted and named pP43H-D30 / E29X, and then transformed int...
Embodiment 3
[0034] Example 3 Verification of High Secretion Ability Asparaginase Production Strain
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com