Signal peptide capable of improving secretion efficiency, and applications thereof
A signal peptide and high-efficiency technology, applied in the field of signal peptides, can solve the problems of low L-asparaginase content, complex extraction process, and low L-asparaginase output, so as to improve secretion capacity, reduce production costs, Effect of Enzyme Production Ability Improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 A novel signal peptide
[0026] The nucleotide sequence of the signal peptide (WapA-SP) provided by the present invention is shown in SEQ ID NO.1: ATGAAAAAAAGAAAGAGGCGAAACTTTAAAAAGGTTCATTGCAGCATTTTTAGTGTTGGCTTTAATGATTTCATTAGTGCCAGCCGATGTACTAGCAAAAATCTACA or is modified from the sequence of SEQ ID NO.1, but the signal peptide function is still performed and the secretion efficiency does not occur A fundamentally altered nucleotide sequence. The above-mentioned signal peptide sequence is obtained by chemical total synthesis or by PCR method.
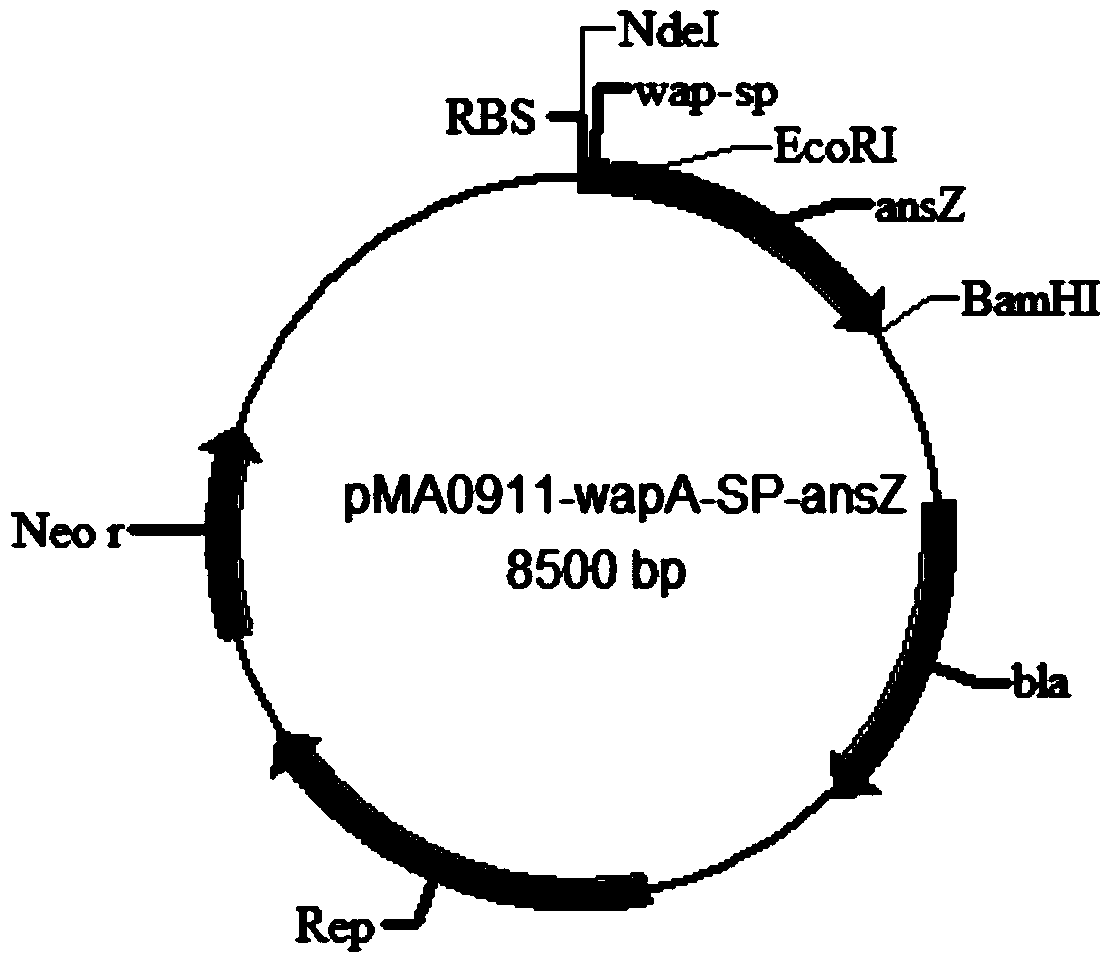
[0027] The above target signal peptide WapA-SP was cloned into the vector pMA5 to construct the plasmid pMA0911-wapA-SP, and the transformant was sequenced by Shanghai Sangon after the enzyme digestion was verified to be correct.
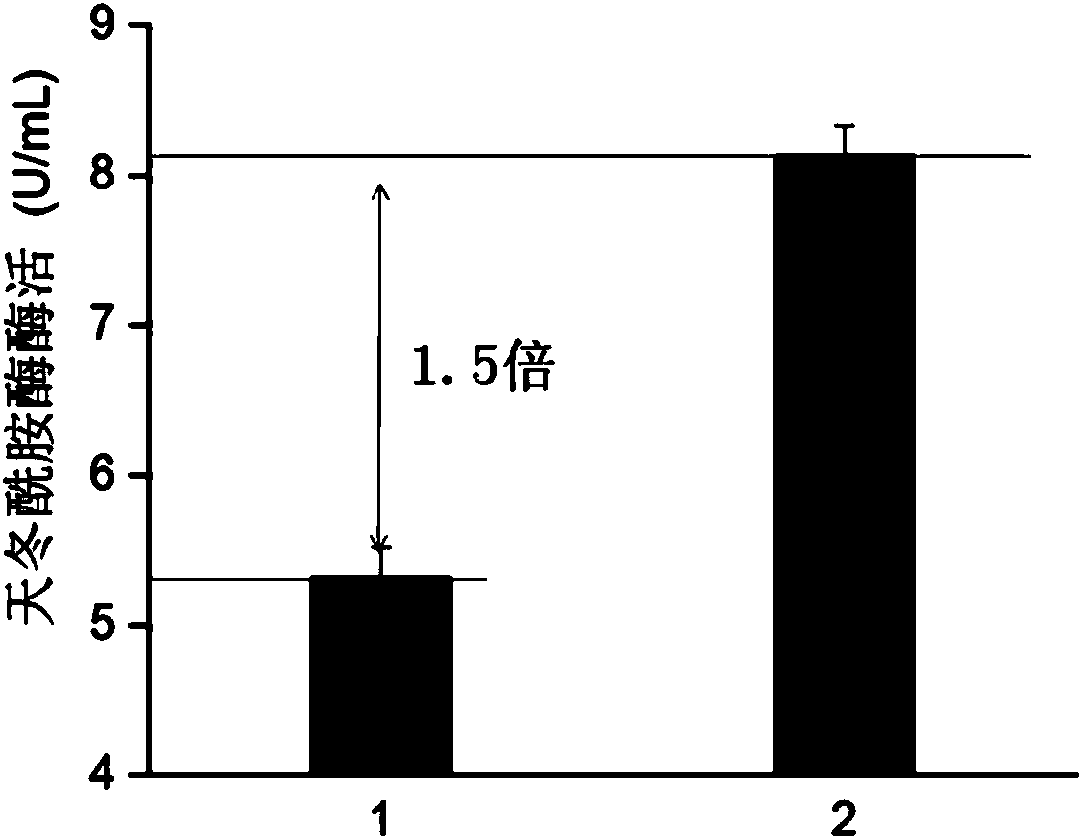
[0028] In this study, by fusing the signal peptide, the secretion pathway of asparaginase is changed to SEC secretion mode, which can increase the secretion of asparaginase and further improve ...
Embodiment 2
[0029] Embodiment 2 asparaginase gene acquisition
[0030] The PCR primers G19Sence and G19Antisence of L-asparaginase gene were designed according to the ansZ gene sequence in the NCBI whole genome nucleic acid sequence of Bacillus subtilis. The ansZ gene lacking the signal peptide (19 N-terminal amino acids) was amplified by PCR.
[0031] G19Sence:CCG GAATTC TGTTCACATTCTCCTGAAACAA (EcoR I)
[0032] G19Antisence:CGC GGATCC TCAATACTCATTGAAATAAGCTTG (BamH I)
[0033] Using pET22b-ansZ (Shanghai Sangong Synthetic) as a template, use the primers provided above for PCR amplification, PCR conditions: 98°C pre-denaturation, 3min, one cycle; 98°C denaturation, 30s, 50°C annealing, 30s, 72 ℃ extension, 70s, 34 cycles; 72°C, 10min, one cycle; 12°C, 10min, one cycle. PCR amplification system: template 1 μL, upstream and downstream primers 1 μL, dNTP Mix 4 μL, 5×primeSTAR Buffer 10 μL, sterilized double distilled water 32.5 μL, primeSTAR DNA polymerase 0.5 μL. The gel recovery ki...
Embodiment 3
[0034] Example 3 High-efficiency secretion of asparaginase strain construction
[0035] The target gene ansZ was ligated with the vector pMA0911-wapA-SP, the ligation system: 4 μL of the target gene, 1 μL of the vector pMA0911-wapA-SP, 5 μL of solution I, ligated overnight at 16°C. Transform the ligated recombinant plasmid pMA0911-wapA-SP-ansZ into competent E.coil JM109, and use ampicillin LB plates to pick positive colonies. The plasmid was extracted after overnight culture on a shaker at 37°C and named pMA0911-wapA-SP-ansZ. After the enzyme digestion was verified to be correct, the transformants were sequenced by Shanghai Sangon.
[0036] Transform the plasmid with correct sequencing into Bacillus subtilis WB600 to obtain asparaginase-producing genetically engineered bacteria.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com