A signal peptide that can effectively improve the efficiency of protein secretion and expression and its application
A technology for secretion expression and signal peptide, applied in the field of signal peptide, can solve the problem of not being able to achieve better technical effects, and achieve the effect of improving secretion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: A new type of signal peptide
[0043] The nucleotide sequence of the signal peptide provided in the present invention is as shown in SEQ ID NO: 2, which is based on the signal peptide PelB (SEQ ID NO: 4) and OmpA (SEQ ID NO: 5). It was transformed. The N terminal of the signal peptide uses the first three amino acid disabled MKK of the signal peptide OmpA, the middle sequence is the strong hydrophobic sequence LLPTAAAGLLLLAAQP of PelB, and the C terminal uses the last three amino acid residues AQA of OmpA. The signal peptide sequence is obtained by chemical synthesis or PCR, and its usability is analyzed by signalP 3.0.
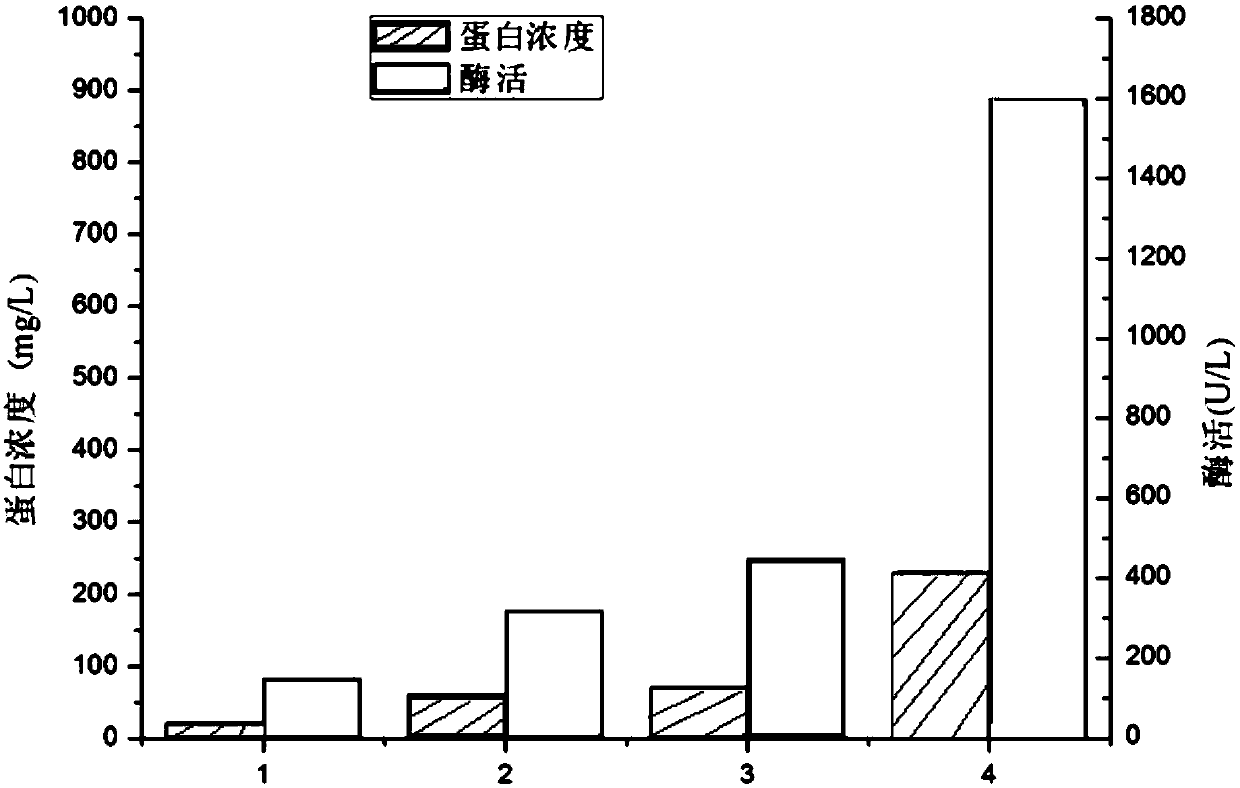
[0044] This signal peptide was cloned into the NcoI restriction site of the vector pET-28a(+), and then the target gene PLD was cloned into the C-terminal of this signal peptide, the secretion of the target protein was effectively enhanced after expression. The secretion of PLD is effectively increased to 0.23g / L, and the extracellular enzyme a...
Embodiment 2
[0045] Example 2: Acquisition of phospholipase D gene.
[0046] The target protein used in the present invention is phospholipase D in Escherichia coli BL21, and its phospholipase D functional gene is obtained by PCR.
[0047] 1. Genomic extraction of E. coli BL21.
[0048] 1) Collect 1ml of bacterial solution in 1.5ml Tube, centrifuge at 12,000rpm for 2 minutes, and discard the supernatant (cell culture solution).
[0049] 2) Add 180μl of Buffer GL, 20μl of Proteinase K (20mg / ml) and 10μl of RNase A (10mg / ml) to mix thoroughly and incubate in a water bath at 56℃ for 10 minutes. At this time, the solution should be transparent and clear. .
[0050] 3) Add 200 μl of Buffer GB and 200 μl of 100% ethanol, and mix thoroughly by pipetting.
[0051] 4) Install the Spin Column on the Collection Tube, transfer the solution to the Spin Column, centrifuge at 12,000 rpm for 2 minutes, and discard the filtrate.
[0052] 5) Add 500 μl of Buffer WA to Spin Column, centrifuge at 12,000 rpm for 1 minute...
Embodiment 3
[0064] Example 3: Construction of a phospholipase D secretory expression strain to induce expression.
[0065] 1. Construction and transformation of recombinant plasmids.
[0066] First, the plasmid pET-28a was linearized with EcoRI and NdeI, and purified and recovered. The PCR product and linearized plasmid are ligated by one-step cloning method. The system is as follows: PCR purified product 2ul, linearized pET-28a plasmid 4ul, CE buffer 4ul, ExnaseⅡ 2ul, deionized water 8ul, react at 37℃ for 30 minutes , Construct the recombinant plasmid pET-28a-PLD. Then the above plasmid pET-28a-PLD was treated with NcoI endonuclease. Finally, the synthesized signal peptide sequence and linearized pET-28a-PLD are ligated by one-step cloning method to construct the final plasmid pET-28a-sp-PLD ( Figure 4 ). Send Jin Weizhi sequencing.
[0067] 2. Induced expression of recombinant bacteria.
[0068] The secretion expression plasmid pET-28a-sp-PLD constructed above was transferred into the Rose...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com