Modified i(E.coli) enterotoxin II signal peptide and microorganism expressing fusion protein of said peptide and heterologous protein
A heterologous protein, E. coli technology, applied in microorganisms, animal/human proteins, microorganism-based methods, etc., can solve problems such as low hGH production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The following examples are intended to further illustrate the invention without limiting its scope. Preliminary Example 1: Screening of human growth hormone cDNA gene
[0065] (Step 1) Construction of human pituitary cDNA library
[0066] To 1 g of human pituitary gland was added 10 ml of guanidine solution (4M guanidine isothiocyanate, 50 mM Tris-HCl, pH 7.5, 10 mM EDTA, and 5% 2-mercaptoethanol) and homogenized. The homogenate was centrifuged at 10000 rpm for 10 minutes at 6°C. 1 / 10 volume of 2% sarkosyl ether (Sigma, USA) was added to the supernatant, and the mixture was kept at 65°C for 2 minutes. Cesium chloride was added to the resulting solution to a final concentration of 0.1 g / ml, and the mixture was centrifuged at 25,000 rpm for 16 hours on 9 ml of a cushion solution (5.7M CsCl and 0.1 mM EDTA) to obtain an RNA pellet. The precipitate was dissolved in 3ml of suspension (5mM EDTA, 0.5% sarkosyl and 5% mercaptoethanol), and then washed successively with pheno...
Embodiment 2
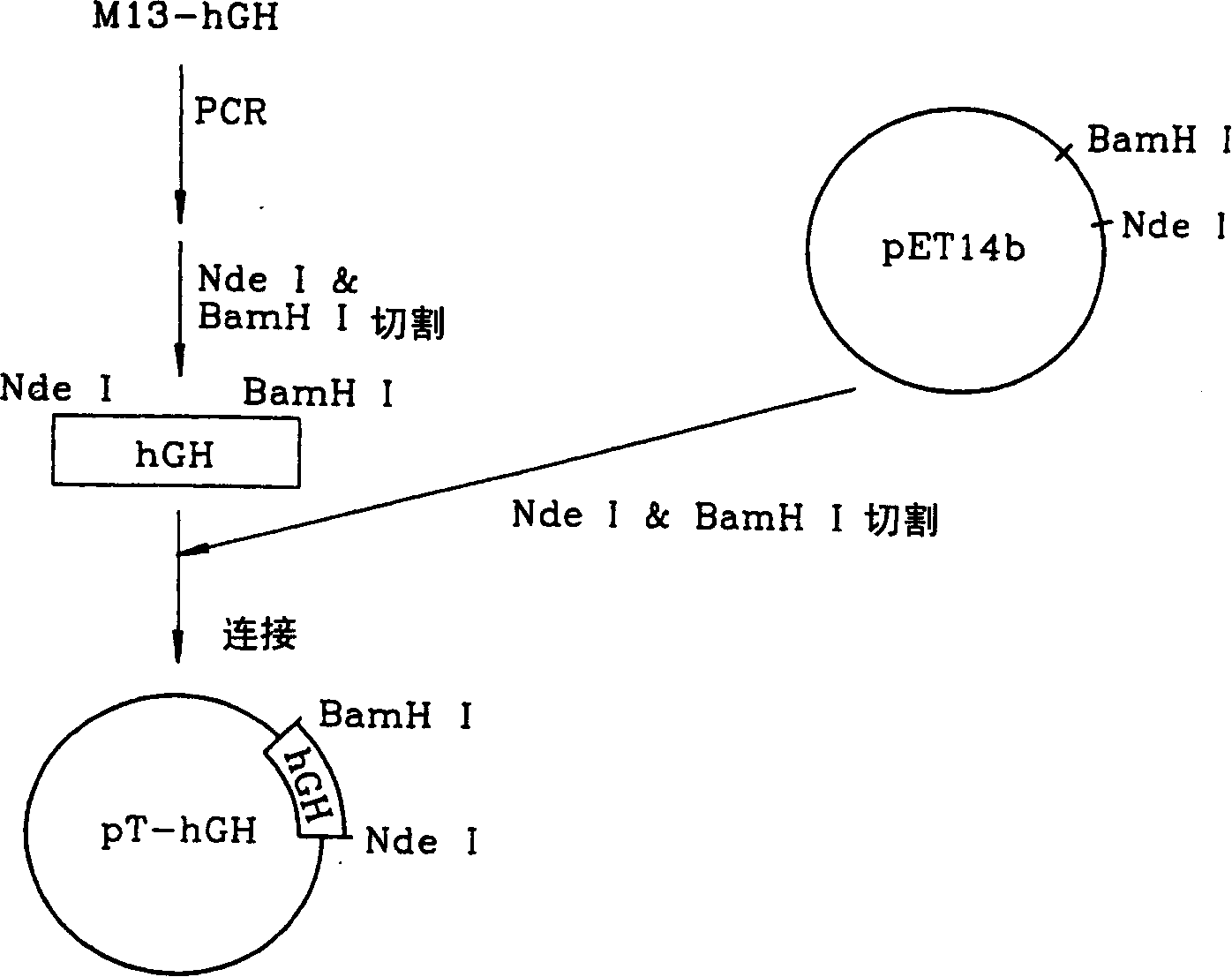
[0077] In order to confirm that the clone contains the human growth hormone gene, the cloned phage DNA was cut with EcoRI, and then the DNA fragment was subjected to Southern blot using a mixed sequence oligonucleotide probe (Southern, E., Journal of Molecular Biology 98:503 (1975 )). In addition, a 0.65 kb EcoRI fragment containing human growth hormone gene was inserted into the EcoRI site of M13mp18 vector (Pharmacia, USA) to obtain vector M13-hGH. The nucleotide sequence of the human growth hormone gene in the vector M13-hGH was determined by the dideoxy-mediated chain termination method (Sanger, F. et al., Proc. Acad. Sci. USA 74:5463-5467 (1977)). Preliminary Example 2: Preparation of Gene Encoding Mature Human Growth Hormone
[0078] To prepare a cDNA gene encoding mature human growth hormone, the vector M13-hGH obtained in Step 2 of Preliminary Example 1 was subjected to PCR using the following primers S1 and AS1. The sense primer S1 was designed to provide an NdeI re...
Embodiment 3
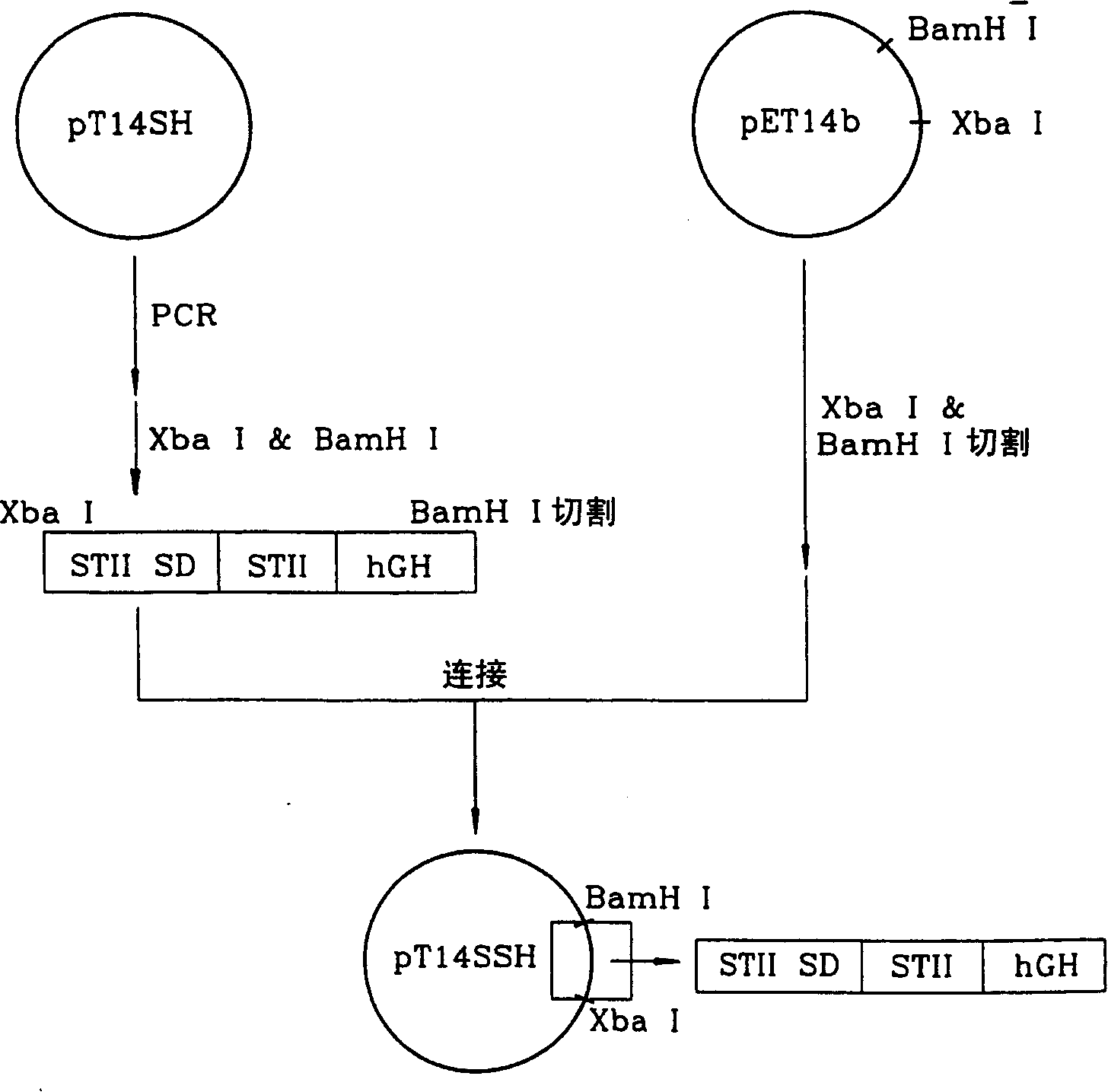
[0084] figure 1 The procedure for constructing the vector pT-hGH above is shown. Preliminary Example 3: Construction of a Vector Containing a Gene Encoding Escherichia coli Endotoxin II Signal Peptide / hGH Fusion Protein
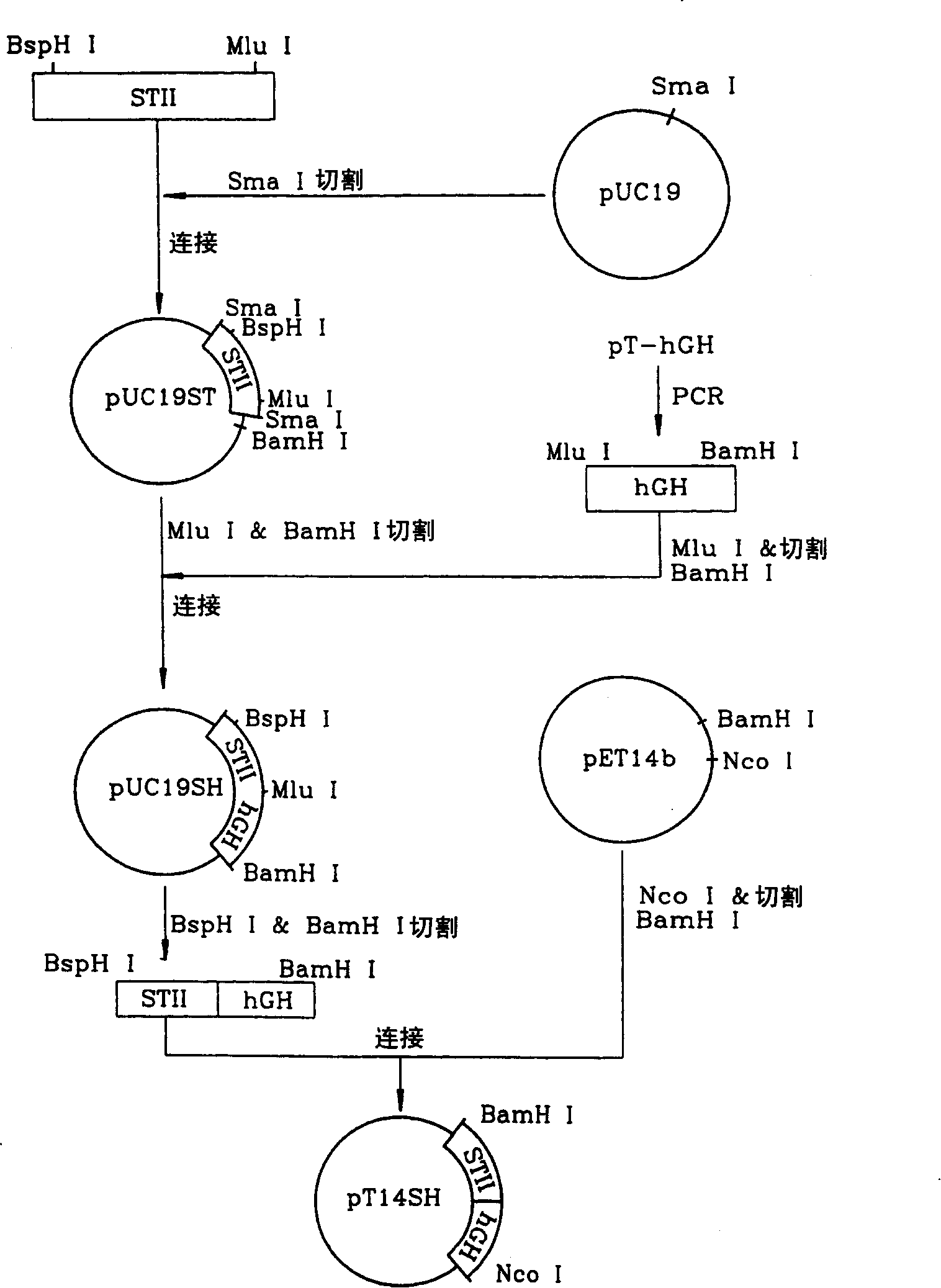
[0085] (Step 1) Cloning the Escherichia coli endotoxin II signal peptide gene
[0086] In order to prepare the Escherichia coli endotoxin II signal peptide gene, design the following pair of complementary oligonucleotides based on the nucleotide sequence of the Escherichia coli endotoxin II signal peptide, and use a DNA synthesizer (type 380B, Applied Biosystem, USA) synthesis.
[0087] Sense strand oligonucleotide STⅡS1 (SEQ ID NO:8) 5'-TCATGAAAAAGAATATCGCATTTCTTCTTGCATCTATGTTCGTTTTTCTATTGCTACAAATGCCTACGCGT-3'
[0088] Antisense strand oligonucleotide STⅡ AS1 (SEQ ID NO:9)
[0089] 5'-ACGCGTAGGCATTTGTAGCAATAGAAAAAACGAACATAGATGCAAGAAGAAATGCGATATTTCTTTTTCATGA-3'
[0090] The oligonucleotide was designed to have NcoI and BspHI restriction sites upstream of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com