Asparaginase mutant with enhanced enzyme activity
An asparaginase and asparagine technology, applied in the field of enzyme engineering, can solve the problems of complex extraction process, low L-asparaginase content, low L-asparaginase yield, etc. The effect of improving enzyme production capacity and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 High-efficiency secretion of asparaginase strain construction
[0021] Using NdeI and BamHI as restriction endonuclease sites, carry out double enzyme digestion on the plasmid pMA0911-wapA-SP-ansZ (fragment) and pET22b(+) plasmid (vector), use the gel recovery kit to digest The plasmid was purified and recovered, and the concentration of the recovered product was checked by electrophoresis. The recovered target gene (wapA-SP-ansZ) was ligated with the carrier pET22b(+), the connection system: 4 μL of the target gene (wapA-SP-ansZ), 1 μL of the vector (pET22b(+)), 5 μL of solutionI, overnight at 16°C . Transform the ligated recombinant plasmid pET22b-wapA-SP / ansZ into competent E.coil JM109, transform it into an ampicillin LB plate, and pick positive colonies. Such as figure 1 . The plasmid was extracted after overnight culture on a shaker at 37°C and named pET22b-wapA-SP / ansZ. After the enzyme digestion was verified to be correct, the transformants were se...
Embodiment 2
[0023] Example 2 Verification of high secretion capacity asparaginase production strain
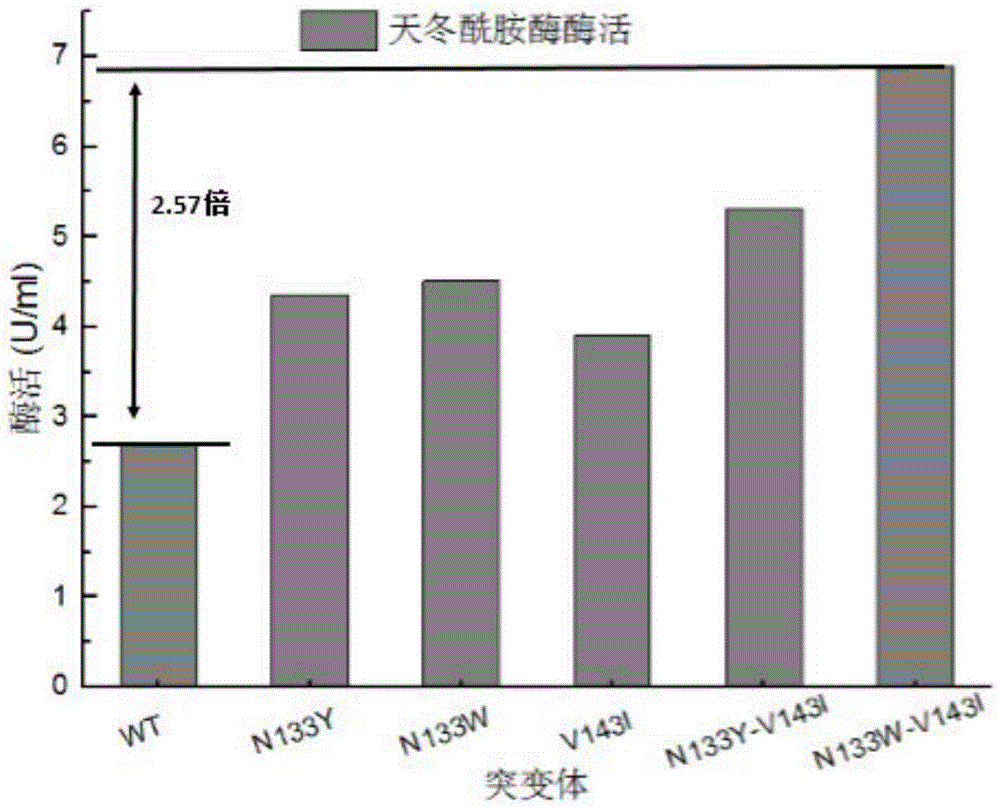
[0024] The plasmid sequenced correctly in Example 1 was transformed into Escherichia coli E. coli rosetta. Selected transformants were inoculated into LB liquid medium, cultured at 37°C for 12 hours, and then transferred into TB medium with an inoculation amount of 3%. Bacteria grow to OD 600 When it was 1.5, IPTG was added to induce, and the culture temperature was lowered to 30°C, and cultured for 24h. The fermentation supernatant was collected, and the enzyme activity of the fermentation supernatant was detected, and the results showed that asparaginase was secreted extracellularly. The enzyme activity is 2.68U / ml.
Embodiment 3
[0025] Embodiment 3 Obtaining of high activity and thermostable mutant strain
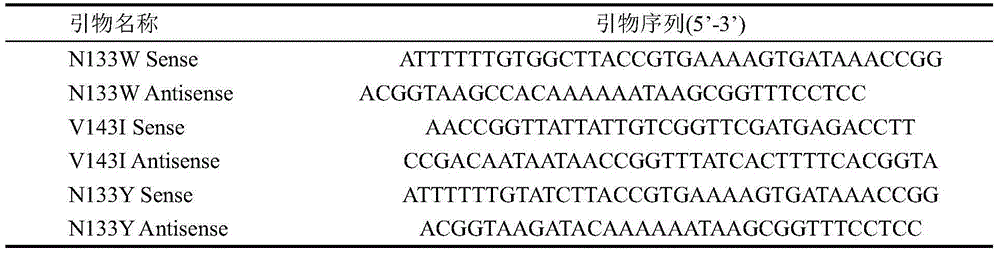
[0026] Using the site-directed mutagenesis kit (TaKaRa), design 3 pairs of primers (as shown in Table 1), and use the constructed pET22b-wapA-SP / ansZ as a template to carry out PCR, and the asparagine at position 133 inside the asparaginase molecule They were mutated into tyrosine and tryptophan, respectively, and valine at position 143 was mutated into isoleucine, named N133Y, N133W, and V143I, respectively. The PCR reaction conditions were: 95° C. for 5 min, 34 cycles (95° C. for 5 min, 60° C. for 30 s, 72° C. for 5 min and 40 s), and 72° C. for 10 min. PCR amplification system: template 1 μL, upstream and downstream primers 1 μL, dNTP Mix 4 μL, 5×primeSTAR Buffer 10 μL, sterilized double distilled water 32.5 μL, primeSTAR DNA polymerase 0.5 μL. The gel recovery kit was used to purify and recover the PCR product, and the concentration of the recovered product was checked by electrophoresis. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com